LA DERMATITIS SEBORREICA Y SUS ALTERNATIVAS TERAPÉUTICAS, ACTUALIZACIÓN.
THE SEBORRHEIC DERMATITIS AND THERAPEUTIC ALTERNATIVES, A REVIEW.
DERMATITIS SEBORREICA MEJILLAS
PUBLICADO 2017 ACTUALIZADO 2025
===================
Hola amigos de la red, DERMAGIC EXPRESS de nuevo, hoy con el interesante tema: LA DERMATITIS SEBORREICA, una enfermedad no tan severa pero muy frecuente hoy en dia en la población, tanto niños como adultos, hombres y mujeres.
2.) SÍNTOMAS CLÍNICOS:
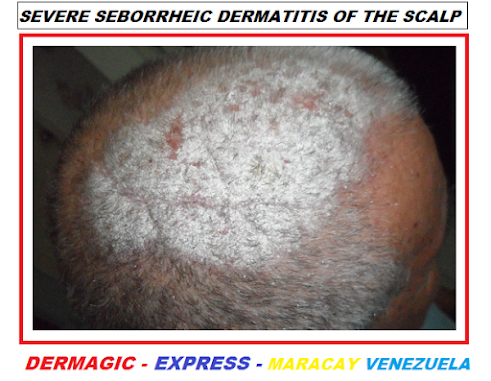
A - Cuero cabelludo: la línea de implantación del cabello, llamada corona seborréica, pero todo el cuero cabelludo puede estar afectado.
B - Región facial media: cejas, surcos nasogenianos, alas de la nariz.
F - Área periglútea: pliegue interglúteo.
G - Párpados y borde palpebral: a veces afectando las pestañas, conocida con el nombre de BLEFARITIS SEBORREICA.
Estas zonas se caracterizan por presentar piel grasa, o con secreción sebácea abundante, lo cual favorece el desarrollo de la enfermedad.
DERMATITIS SEBORREICA DE LA CARA
El Curso de la DERMATITIS SEBORREICA es crónico y recurrente, con períodos de remisión y exacerbación.
DERMATITIS SEBORREICA SEVERA DEL CUERO CABELLUDO
3.) ÁCIDO SALICÍLICO.
4.) PROPILENGLICOL.
5.) PIRITIONATO DE ZINC.
6.) BIOTINA.
7.) KETOCONAZOL.
8.) FLUCONAZOL.
9.) SERTACONAZOL.
10.) ITRACONAZOL.
11.) DITRANOL.
12.) SUCCINADO DE LITIO.
13.) VITAMINA-COMPLEJO B.
14.) PEROXIDO DE BENZOILO.
15.) SULFURO DE SELENIO.
16.) SULFACETAMIDA.
17.) TACROLIMUS y 18.) PIMECROLIMUS (Hoy día 2025 estos medicamentos tienen una advertencia severa por la FDA tipo "BLACK BOX" o "CAJA NEGRA", en sus enpaques acerca de que el prolongado puede inducir malignidad)
18.) PIMECROLIMUS.
19.) METRONIDAZOL.
20.) TERBINAFINA.
21.) ALQUITRAN DE PINO.
22.) ALQUITRAN DE HULLA.
23.) GLICERINA.
24.) TACALCITOL.
25.) FOTOTERAPIA y FOTOQUIMIOTERAPIA
26.) AZUFRE.
27.) BIFONAZOL.
28.) CICLOPIROXOLAMINA.
29.) L - DOPA.
===================
Hello friends of the network, DERMAGIC EXPRESS is back again, today with the interesting topic: SEBORRHEIC DERMATITIS, a disease that is not very severe but very common nowadays in the population, affecting both children and adults, men and women.
1.) HISTORY:
SEBORRHEIC DERMATITIS was first described in 1732 by Paul Gerson Unna, a German physician and one of the pioneers in dermatology.
In 1873, the Italian physician Giuseppe Rivalta suggested a possible fungal origin for SEBORRHEIC DERMATITIS.
In 1874, Louis-Charles Malassez, a physician from France, linked the presence of yeasts called Malassezia to seborrheic dermatitis. He identified fungal structures on the scalp, which he called "champignon de la pelade" ("baldness fungus"), and associated them with pityriasis capitis (a condition linked to seborrheic dermatitis).
His contribution was significant and arguably key in discovering the presence of these yeasts on the skin and their probable role in some dermatological diseases. Because of this, the genus of these yeasts was named "Malassezia" in his honor.
In 1952, Riccardo Galeazzi Leone, also an Italian physician, discovered the association between PITYROSPORUM OVALE (now MALASSEZIA FURFUR) and this disease.
Regarding the fungus MALASSEZIA FURFUR, it was discovered in 1846 by the German gynecologist and dermatologist Karl Ferdinand Eichstedt, and was associated with PITYRIASIS VERSICOLOR, also a skin condition; and later it was also linked to SEBORRHEIC DERMATITIS, as we just mentioned.
2.) CLINICAL SYMPTOMS:
The disease primarily affects the face and scalp, and less frequently the anterior and posterior chest.
Characterized by itchy, scaly, erythematous plaques, seborrheic dermatitis is a chronic condition that presents a significant treatment challenge due to its frequent relapse, even with the best treatment options.
The most affected areas of the body are the so-called "seborrheic zones," which are areas with the highest concentration of sebaceous glands and where seborrheic dermatitis typically appears. These include:
A - Scalp: the hairline, called the seborrheic corona, but the entire scalp can be affected.
B - Midface: eyebrows, nasolabial folds, and sides of the nose.
C - Mid-chest and interscapular region of the back: between the shoulder blades.
D - Behind the ears and external auditory canals.
E - Axillary, inframammary, and inguinal folds: less common.
F - Perigluteal area: intergluteal fold.
G - Eyelids and eyelid margins: sometimes affecting the eyelashes, known as SEBORRHEIC BLEPHARITIS.
These areas are characterized by oily skin, or abundant sebum secretion, which favors the development of the disease.
On the scalp, the flaking caused by SEBORRHEIC DERMATITIS is commonly known as "DANDRUFF", perhaps one of the most frequent reasons for dermatological consultations.
The course of seborrheic dermatitis is chronic and recurrent, with periods of remission and exacerbation.
It can begin in childhood, and it is most common in young adults and older adults.
Outbreaks worsen with stress, cold or very hot weather, and underlying diseases (e.g., HIV, Parkinson's). In rare cases, it can become generalized in some patients, but it is usually limited to the seborrheic areas already described.
4.) TREATMENTS:
A.- 1950s-1960s: Topical corticosteroids began to be used to reduce inflammation. The use of low-potency topical corticosteroids (hydrocortisone), sulfacetamide, and sulfur to reduce itching, erythema, and inflammation is still current.
B. In the 1970s and 80s: the use of antifungals such as KETOCONAZOLE, FLUCONAZOLE, BIFONAZOLE, ITRACONAZOLE, SERTACONAZOLE, MICONAZOLE, TERBINAFINE and CYCLOPRIROXOLAMINE was FORMALLY introduced to control the MALASSEZIA yeast. This was somewhat late, as the relationship between this fungus and seborrheic dermatitis was practically established in the 1950s.
- In the 1980s (1985), ITRACONAZOLE was specifically introduced.
- In the 1990s, METRONIDAZOLE began to be used in topical gel form.
C.- From the 1990s to 2000: the additional use of keratolytic agents and tars (cade oil, urea) was introduced to improve scaling and promote skin regeneration.
- In the 2000s, TACROLIMUS and PIMECROLIMUS emerged as therapeutic alternatives.
D.- 2000-2025: Combinations of the above were introduced: topical antifungals with low-potency corticosteroids, and MAGISTRAL FORMULATIONS with fluid biosulfur, ciclopiroxolamine, and other components, prepared as shampoos and creams for topical use.
2.) UREA.
3.) SALIYLIC ACID.
4.) PROPYLENE-GLYCOL.
5.) PYRITHIONE ZINC.
6.) BIOTIN.
7.) KETOCONAZOLE.
8.) FLUCONAZOLE.
9.) SERTACONAZOLE.
10.) ITRACONAZOLE.
11.) DITHRANOL.
12.) LITHIUM SUCCINATE.
13.) VITAMIN-COMPLEX B.
14.) BENZOYL PEROXIDE.
15.) SELENIUM SULFIDE.
16.) SULFACETAMIDE.
17.) TACROLIMUS. 18.) PIMECROLIMUS, (Today 2023 these drugs TACROLIMUS AND PIMECROLIMUS have a severe warning from the FDA that their prolonged use can induce cancer)
18.) PIMECROLIMUS.
19.) METRONIDAZOLE.
20.) TERBINAFINE.
21.) PINE TAR.
22.) COALTAR.
23.) GLYCERINE.
24.) TACALCITOL.
25.) PHOTOTHERAPY.
26.) SULFUR.
27.) BIFONAZOLE.
28.) CYCLOPYROXOLAMINE.
29.) L-DOPA.
5.) NEW TREATMENTS:
- ROFLUMILAST: is a phosphodiesterase-4 (PDE4) inhibitor whose mechanism of action is to reduce skin inflammation.
- It is a 0.3% topical foam which was approved on December 16, 2023, by the FDA for the treatment of seborrheic dermatitis in adults and children over 9 years of age.
- It is the first medication approved in more than two decades, meaning since the 1980s, to treat this condition; its main effect is to control redness and skin inflammation.
Otherwise, the well-known commercial creams and shampoos continue to be used, with MAGISTRAL FORMULATIONS remaining excellent options for these cases, such as shampoos prepared with sulfur, salicylic acid, and hair lotions, interspersed with other commonly used products.
Seborrheic dermatitis is often recalcitrant and difficult to treat, as many patients relapse after showing improvement. Excess oil on the face and stress are major triggers and causes of relapse.
Dr. Jose Lapenta.
REFRENCIAS BIBLIOGRÁFICAS / BIBLIOGRAPHICAL REFERENCES
=============================================================
C.- Roflumilast and the Changing Landscape of Seborrheic Dermatitis Treatment (2025).
2.) Treatment of seborrheic dermatitis: comparison of sertaconazole 2 % cream versus pimecrolimus 1 % cream.
3.) Single-blind, randomized controlled trial evaluating the treatment of facial seborrheic dermatitis with hydrocortisone 1% ointment compared with tacrolimus 0.1% ointment in adults.
4.) New strategies in dandruff treatment: growth control of Malassezia ovalis.
5.) Narrow-band ultraviolet B (ATL-01) phototherapy is an effective and safe treatment option for patients with severe seborrhoeic dermatitis.
6.) Four cases of sebopsoriasis or seborrheic dermatitis of the face and scalp successfully treated with 1a-24 (R)-dihydroxycholecalciferol (tacalcitol) cream.
7.) High prevalence of seborrhoeic dermatitis on the face and scalp in mountain guides.
8.) The antifungal action of dandruff shampoos.
9.) Treatment of scalp seborrheic dermatitis and psoriasis with an ointment of 40% urea and 1% bifonazole.
10.) [Effect of anti-seborrhea substances against Pityrosporum ovale in vitro].
11.) Facial seborrheic dermatitis treated with fluconazole 2% shampoo.
12.) Pityrosporum ovale and skin diseases.
13.)[Therapy of seborrheic eczema with an antifungal agent with an antiphlogistic effect].
14.) [A case of seborrhoeic blepharitis].
15.) Treatment of sebopsoriasis with itraconazole.
16.) Treatment of seborrheic dermatitis.
17.) Pityrosporum ovale (Malassezia furfur) as the causative agent of seborrhoeic dermatitis: new treatment options.
18.) Relation Between Skin Temperature and Location of Facial Lesions in Seborrheic Dermatitis
19.) Insulin Quantification in Patients With Seborrheic Dermatitis
20.) Humoral immunity to Malassezia furfur serovars A, B and C in patients with pityriasis versicolor, seborrheic dermatitis and controls.
21.) Management of common superficial fungal infections in patients with AIDS.
22.) Pityrosporum infections.
23.) Seborrheic dermatitis as a revealing feature of HIV infection in Bamako, Mali [letter]
24.) Cell-mediated immune responses to Malassezia furfur serovars A, B and C in patients with pityriasis versicolor, seborrheic dermatitis and controls.
25.) The efficacy of 1% metronidazole gel in facial seborrheic dermatitis: a double blind study.
26.) A double blind study of the effectiveness of sertaconazole 2% cream vs. metronidazole 1% gel in the treatment of seborrheic dermatitis.
27.) Pimecrolimus 1% cream, methylprednisolone aceponate 0.1% cream and metronidazole 0.75% gel in the treatment of seborrhoeic dermatitis: a randomized clinical study.
28.) Treatment with bifonazole shampoo for seborrhea and seborrheic dermatitis: a randomized, double-blind study.
29.) Quantitative skin cultures of Pityrosporum yeasts in patients seropositive for the human immunodeficiency virus with and without seborrheic dermatitis.
30.) A double-blind, placebo-controlled, multicenter trial of lithium succinate ointment in the treatment of seborrheic dermatitis. Efalith Multicenter Trial Group.
31.) Ketoconazole 2% emulsion in the treatment of seborrheic dermatitis.
32.) Seborrheic dermatitis in acquired immunodeficiency syndrome.
33.) Blood levels of vitamin E, polyunsaturated fatty acids of phospholipids, lipoperoxides and glutathione peroxidase in patients affected with seborrheic dermatitis.
34.) Skin surface lipids in HIV sero-positive and HIV sero-negative patients affected with seborrheic dermatitis.
35.) Seborrheic dermatitis and daylight [see comments]
36.) [Seborrheic dermatitis and cancer of the upper
respiratory and digestive tracts]
37.) The role of Pityrosporum ovale in seborrheic dermatitis.
38.) Correlation of Pityosporum ovale density with clinical severity of seborrheic dermatitis as assessed by a simplified technique.
39.) Immune reactions to Pityrosporum ovale in adult
patients with atopic and seborrheic dermatitis.
40.) [Treatment of seborrheic dermatitis with benzoyl peroxide]
41.)[The significance of yeasts in seborrheic eczema]
42.) Association of Pityrosporum orbiculare (Malassezia furfur) with seborrheic dermatitis in patients with acquired immunodeficiency syndrome (AIDS).
43.) Pityrosporum ovale in infantile seborrheic dermatitis.
44.)Infantile seborrheic dermatitis: seven-year follow-up and some prognostic criteria.
45.) Ketoconazole 2% cream versus hydrocortisone 1% cream in the treatment of seborrheic dermatitis. A double-blind comparative study.
46.) T-cell subset assay. A useful differentiating marker of atopic and seborrheic eczema in infancy?
47.) Propylene glycol in the treatment of seborrheic dermatitis of the scalp: a double-blind study.
48.) Seborrheic dermatitis and malignancy. An investigation of the skin flora.
49.) Efficacy of topical application of glucocorticosteroids compared with eosin in infants with seborrheic dermatitis.
50.) Erythema with features of seborrheic dermatitis and lupus erythematosus associated with systemic 5-fluorouracil.
51.)[Treatment of seborrheic dermatitis with low-dosage dithranol]
52.) Double-blind treatment of seborrheic dermatitis with 2% ketoconazole cream.
53.) Seborrheic dermatitis in neuroleptic-induced parkinsonism.
54.) Successful treatment and prophylaxis of scalp seborrhoeic dermatitis and dandruff with 2% ketoconazole shampoo: results of a multicentre, double-blind, placebo-controlled trial.
55.) Adherence of Malassezia furfur to human stratum corneum cells in vitro: a study of healthy individuals and patients with seborrhoeic dermatitis.
56.) Seborrhoeic dermatitis: treatment with anti-mycotic agents.
57.)Analyses of skin surface lipid in patients with microbially associated skin disease.
58.) Borage oil, an effective new treatment for infantile seborrhoeic dermatitis [letter]
59.) Transepidermal water loss and water content in the stratum corneum in infantile seborrhoeic dermatitis.
60.) [Skin lipids in seborrhea- and sebostasis-associated skin diseases]
61.) Use of topical lithium succinate in the treatment of seborrhoeic dermatitis [letter; comment]
62.) A dose-response study of irritant reactions to sodium lauryl sulphate in patients with seborrhoeic dermatitis and atopic eczema.
63.) Seborrhoeic dermatitis of the scalp--a manifestation of Hailey-Hailey disease in a predisposed individual?
64.) Use of topical lithium succinate in the treatment of seborrhoeic dermatitis [see comments]
65.) Erythema multiforme and dermatitis seborrhoides infantum as concomitant id-reactions to widespread candidosis in a suckling.
66.) The evaluation of various methods and antigens for the detection of antibodies against Pityrosporum ovale in patients with seborrhoeic dermatitis.
67.) Enhanced phagocytosis and intracellular killing of Pityrosporum ovale by human neutrophils after exposure to ketoconazole is correlated to changes of the yeast cell surface.
68.) [Therapy of seborrheic eczema with an antifungal agent with an antiphlogistic effect]
69.) Neutrophil zinc levels in psoriasis and seborrhoeic dermatitis.
70.) Skin surface electron microscopy in Pityrosporum folliculitis. The role of follicular occlusion in disease and the response to oral ketoconazole.
71.) Studies on the yeast flora in patients suffering from psoriasis capillitii or seborrhoic dermatitis of the scalp.
72.) [Histological differential diagnosis of psoriasis vulgaris and seborrheic eczema of the scalp]
73.) Tinea versicolor with regard to seborrheic dermatitis. An epidemiological investigation.
74.)Quantitative microbiology of the scalp in non-dandruff, dandruff, and seborrheic dermatitis.
75.) Treatment of seborrheic dermatitis with biotin and vitamin B complex.
76.) L-dopa for seborrheic dermatitis.
77.) Seborrheic dermatitis of infants: treatment with biotin injections for the nursing mother.
78.) Photochemotherapy in erythrodermic seborrhoic dermatitis [letter]
79.) Old drug--in a new system--revisited.
80.) Oral use of biotin in seborrhoeic dermatitis of infancy: a controlled trial.
81.) Generalized seborrhoeic dermatitis. Clinical and therapeutic data of 25 patients.
82.)The effect of betamethasone valerate on seborrhoeic dermatitis of the scalp. A clinical, histopathological cell kinetic study.
83.) Topical glycerin in seborrhoeic dermatitis.
84.)[Therapeutic aspects of seborrhea oleosa and pityriasis simplex capillitii]
85.) Tinea versicolor and Pityrosporum orbiculare: mycological investigations, experimental infections and epidemiological surveys.
86.)[Some atypical forms of eczema in children (author's transl)]
87.) Efficacy and safety of a low molecular weight hyaluronic Acid topical gel in the treatment of facial seborrheic dermatitis final report.
88.) Treatment of seborrheic dermatitis: the efficiency of sertaconazole 2% cream vs. tacrolimus 0.03% cream.
89.) Comparison the efficacy of fluconazole and terbinafine in patients with moderate to severe seborrheic dermatitis.
90.) Efficiency of terbinafine 1% cream in comparison with ketoconazole 2% cream and placebo in patients with facial seborrheic dermatitis.
91.) A novel cosmetic antifungal/anti-inflammatory topical gel for the treatment of mild to moderate seborrheic dermatitis of the face: a open-label trial utilizing clinical evaluation and erythemadirected digital photography.
92.) Treatment of moderate to severe facial seborrheic dermatitis with itraconazole: an open non-comparative study.
93.) Role of antifungal agents in the treatment of seborrheic dermatitis.
94.) Investigations of seborrheic dermatitis. Part II. Influence of itraconazole on the clinical condition and the level of selected cytokines in seborrheic dermatitis.
95.) Efficacy and Safety of Cream Containing Climbazole/Piroctone Olamine for Facial Seborrheic Dermatitis: A Single-Center, Open-Label Split-Face Clinical Study.
96.) Topical Treatment of Facial Seborrheic Dermatitis: A Systematic Review.
97.) Low-dose oral isotretinoin for moderate to severe seborrhea and seborrheic dermatitis: a
randomized comparative trial.
98.) Effect of itraconazole on the quality of life in patients with moderate to severe seborrheic dermatitis: a randomized, placebo-controlled trial.
99.) Zinc Pyrithione: A Topical Antimicrobial With Complex Pharmaceutics.
100.) Topical pine tar: History, properties and use as a treatment for common skin conditions.