Marburg Virus, y Los murciélagos. !
Marburg Virus, and the bat !
EDITORIAL ESPAÑOL
=================
Hola amigos de la red DERMAGIC EXPRESS te trae hoy un tema bien interesante,
se trata de EL VIRUS MARBURG(MARV), LA NOCHE DE LOS MURCIELAGOS, virus hoy día considerado UNO DE LOS VIRUS MAS LETALES para la HUMANIDAD, luego del VIRUS EBOLA, aunque ambos pertenecen a la misma familia de FILOVIRUS.
Pero quizá lo más SORPRENDENTE que encontré en esta revisión es que este virus en los años de la
GUERRA FRÍA en la UNIÓN SOVIÉTICA fue uno de los tantos agentes biológicos que trato de ser utilizado
como ARMA BIOLÓGICA, para ser colocado en las ojivas nucleares de los misiles Rusos. Hecho denunciado por el científico Ruso Ken Alibek, quien
trabajó en uno de los laboratorios en los
años 1.983-1.988 para tales efectos y que desertó a
los Estados Unidos de Norteamérica posteriormente y reveló estos secretos de
Estado.
Otro detalle a considerar si te leíste las revisiones previas, es que el hábitat donde vivimos los humanos NO ES TAN INOFENSIVO, estamos rodeados por GARRAPATAS (Enfermedad de Lyme, Powassan, Heartland, Fiebre de Crimea-Congo y otras) INSECTOS (Zika, Dengue, Chikungunya, Fiebre del Nilo, Encefalitis Japonesa,
jamestown canyon virus y otros), RATAS, RATONES (Hantavirus, Peste bubónica, Leptospirosis, Salmonella y otras) TRANSMISORES de virus que son altamente MORTALES o te inoculan una enfermedad que quizá te deje secuelas de por vida. Y
hoy te traigo además de estos, LOS MURCIELAGOS, inofensivos MAMIFEROS VOLADORES que habitan en cuevas, y de actividad
nocturna, RESERVORIOS NATURALES DEL VIRUS DE MARBURG y ÉBOLA considerados hoy día los 2 virus MÁS LETALES para la humanidad con una mortalidad superior al 80%. Luego del contagio.
EL VIRUS MARBURG (MARV) pertenece
al grupo ARN VIRUS,
orden: MONONEGAVIRALES, familia: FILOVIRIDAE,
genus: MARBURG VIRUS,
especie: MARBURG MARBURG VIRUS.
PRIMER BROTE DEL VIRUS DE MARBURG EN EUROPA 1.967
El VIRUS de MARBURG apareció por primera vez en 1.967 en UN
LABORATORIO en las localidades de MARBURG Y FRANKFURT en Alemania y
en Belgrado, Yugoslavia (hoy
Serbia), cuando trabajadores se infectaron por un patógeno desconocido que
provocó manifestaciones HEMORRÁGICAS y alta letalidad. El
agente causal fue aislado e identificado por científicos de la Universidad de MARBURG, Alemania de allí su nombre. Los portadores del virus fueron MONOS VERDES AFRICANOS (Chlorocebus aethiops) infectados que habían sido importados para ser utilizados en la
fabricación de vacunas en el laboratorio alemán HOECHST AG, hoy día
SANOFI-AVENTIS.
Posteriormente aparecieron brotes en el continente Africano:
1.) SUDÁFRICA: En Johannesburgo 1.975
2.) KENYA: Nairobi 1.980, y en la cueva de Kitum 1.987,
3.) RUSIA: en Kosovo en 1.998-1.990.
4.) REPUBLICA DEMOCRATICA DEL CONGO: en Durba y Watsa, 1.998-2.000.
5.) UGANDA: Mina de Kitaka 2.007
y Bosque de Maramagambo 2.008.
6.) HOLANDA Y ESTADOS UNIDOS: 2 casos importados 2.008.
6.) ANGOLA: en
la provincia de Uije 2.004-2.005
7.) UGANDA: en Kabale 2.012.
8.) UGANDA: en 2.014.
La familia de los FILOVIRUS está compuesta por 8 distintas especies: EBOLA VIRUS, LLOVIU VIRUS, MARBURG VIRUS, RESTON VIRUS, SUDÁN VIRUS, TAI VIRUS, BUNDIBUGYO VIRUS y RAVN VIRUS, de estos RAVN VIRUS es considerado un pariente cercano del MARBURG VIRUS Y
TAMBIEN OCASIONA la enfermedad FIEBRE HEMORRÁGICA POR MARBURG VIRUS.
Este virus está considerado por el CDC (Centros de Control y
Prevención de Enfermedades) un patógeno CATEGORÍA A de agente de BIOTERRORISMO en cuya lista están: TULAREMIA, ÁNTRAX, VIRUELA, TOXINA BOTULÍNICA, PESTE BUBÓNICA y
FIEBRES VIRALES HEMORRÁGICAS que incluye los miembros de la familia FILOVIRIDAE (Marburg y Ébola) y de la familia ARENAVIRIDAE (Lassa virus y
Machupo Virus).
La TRANSMISIÓN de MARBURG
VIRUS inicialmente como les comente fue el contacto de HUMANOS con
tejidos de MONOS VERDES AFRICANOS infectados en 1.967;
Posteriormente se comprobó que el MURCIÉLAGO de la FRUTA africano (Rousettus aegyptiacus) ES PORTADOR DEL VIRUS DE MARBURG(MARV).
Se presentaron brotes en Mineros en las CUEVAS de KITAKA en UGANDA, TURISTAS que visitaron la cueva PHYTON en el mismo país, y otras CUEVAS de África: CUEVA KITUM en KENYA. En 2.008 un turista HOLANDES se enfermo con el VIRUS DE MARBURG luego de visitar la cueva PYTHON en
UGANDA, se cree que las heces pulverizadas en el medio ambiente fueron el mecanismo de contagio. Posteriormente se demostró que este MURCIELAGO es el RESERVORIO NATURAL del MARBURG VIRUS, y también del ÉBOLA VIRUS.
Se sospecha que los primates también pueden ser fuente de infección
pero no se ha demostrado que estos sean RESERVORIOS NATURALES.
También se comprobó la transmisión HUMANO-HUMANO a través de del contacto directo con sangre u otros fluidos
corporales como SALIVA, SUDOR, HECES, ORINA, LÁGRIMAS y LECHE MATERNA de pacientes infectados. Incluso el virus se ha encontrado
en LÁGRIMAS, SEMEN, y BIOPSIA HEPÁTICA semanas a meses después de la infección, siendo esto importante
para el monitoreo de pacientes convalecientes. El CONTACTO con los pacientes infectados y la MANIPULACIÓN de
cadáveres también es fuente de infección y por ello existen protocolos
rígidos para la manipulación de los pacientes.
SÍNTOMAS DE LA ENFERMEDAD MARBURG VIRUS (MVD):
================================================
La ENFERMEDAD es denominada FIEBRE HEMORRÁGICA (HF) POR MARBURG VIRUS (MARV), pero hay que resaltar que el VIRUS RAVN además del MARBURG VIRUS es
también agente causal de la misma.
El Periodo de INCUBACIÓN: tiene un promedio de 5-9 días pero puede alcanzar los 21 días. Luego se presenta una fase de GENERALIZACIÓN: (día 1-5) con CEFALEAS, ESCALOSFRIOS, NAUSEAS, VOMITOS, DIARREA, ERUPCIÓN CUTÁNEA, DOLOR ABDOMINAL, MALESTAR GENERAL. Luego se presenta una fase TEMPRANA ORGANICA, (día 5-13) que incluyen DISNEA, EDEMA CONJUNTIVITIS, EXANTEMA, SINTOMAS DEL SNC: ENCEFALITIS,
CONFUSION, DELIRIO, APATIA, AGRESION, luego se presentan los síntomas HEMORRÁGICOS que producen hipovolemia, HECES CON SANFRE, PETEQUIAS, EQUIMOSIS, HEMATEMESIS, HEMORRAGIAS DE
MUCOSAS Y VISCERAL, PERDIDA DE SANGRE EN LOS SITIOS DE VENOPUNCIÓN, en esta fase el paciente toma dos vías: la recuperación o la muerte.
La creencia POPULAR es que el
paciente muere por las HEMORRAGIAS y no es cierto esto. El paciente pasa a la fase TARDIA ORGANICA (día 13 -21) donde entra en estado de CONVALESCENCIA o continua deteriorándose presentando FIEBRE PERSISTENTE, CONVULSIONES, COMA, COAGULACIÓN INTRAVASCULAR
DISEMINADA (CID), DISTURBIOS METABÓLICOS, SHOCK Y MUERTE que usualmente se presenta entre
el día 8-16.
La MORTALIDAD por el MARBUG VIRUS
oscila entre 80-90%, para algunos científicos es el segundo virus más LETAL para la HUMANIDAD luego del ÉBOLA VIRUS.
TRATAMIENTO:
=============
No hay tratamiento específico contra la enfermedad por Marburg
Virus ni VACUNAS disponibles. El tratamiento se basa en mantener los electrolitos,fluidos,
reemplazar la sangre, factores de la coagulación, presión Sanguínea... y rezar mucho para que el paciente logre salvar su vida. ! De
modo que..:
El famoso personaje HOMBRE-MURCIÉLAGO (BATMAN) vive en una CUEVA donde también viven LOS MURCIÉLAGOS, de allí su nombre, y sale de noche a luchar contra los BANDIDOS de ciudad
GOTICA (GOTHAM), esperemos que nunca se infecte con MARBURG VIRUS, pues sería el fin de la película...
MORALEJA: en el caso de MARBURG
VIRUS, el hombre una vez más invadió el hábitat natural del RESERVORIO, LOS MURCIÉLAGOS en las CUEVAS, en busca de metales preciosos,,, y se encontró con la enfermedad y la MUERTE... Y tú, si eres turista y vas a VISITAR UNA CUEVA mira para el techo y evita los MURCIÉLAGOS!
Saludos a todos.
Dr. José Lapenta
EDITORIAL ENGLISH
==================
Hello friends of the DERMAGIC EXPRESS network today brings you a very interesting topic, it is THE MARBURG VIRUS (MARV), THE NIGHT OF BATS , virus today considered ONE OF THE MOST LETHAL VIRUSES for HUMANITY, after the EBOLA VIRUS, although both belong to the same family of FILOVIRUS.
But perhaps the most AMAZING I found in this review is that this virus in the years of the COLD WAR in the SOVIET UNION was one of the many biological agents that I try to be used as a biological weapon, to be placed in the nuclear warheads of the Russian missiles. This fact was denounced by the Russian scientist Ken Alibek, who worked in one of the laboratories in the years 1.983-1.988 for such purposes and desert to the United States of America later and revealed these state secrets.
Another detail to consider if you read previous reviews, is that the habitat where humans live is NOT SO INOFENSIVE, we are surrounded by TICKS (Lyme disease, Powassan, Heartland, Crimea-Congo fever and others) INSECTS (Zika, Dengue , Chikungunya, Nile Fever, Japanese Encephalitis, jamestown canyon virus and others), RATS, MICE (Hantavirus, Bubonic Plague, Leptospirosis, Salmonella and others) virus TRANSMITTERS of diseases that are highly LETHAL or inoculate you with a disease that may leave you sick lifelong . And today I bring you also these, THE BATS, harmless FLYERS MAMMALS that live in caves, and nocturnal activity, NATURAL RESERVOIRS OF THE VIRUSES OF MARBURG and EBOLA considered today the 2 MOST LETHAL viruses for humanity with mortality greater than 80%. After the contagion.
THE MARBURG VIRUS (MARV) belongs to the group: RNA VIRUS, order: MONONEGAVIRALES, family: FILOVIRIDAE, genus: MARBURGVIRUS, species: MARGBURG MARBURGVIRUS.
MARBURG VIRUS appeared for the first time in 1.967 in a LABORATORY in the localities of MARBURG and FRANKFURT in Germany and in Belgrade, Yugoslavia (today Serbia), when workers were infected by an unknown pathogen that provoked HEMORRHAGIC manifestations and high lethality. The causal agent was isolated and identified by scientists from the University of MARBURG, Germany hence its name. The virus carriers were infected AFRICAN GREEN MONKEYS (Chlorocebus aethiops) that had been imported to be used in the manufacture of vaccines in the German HOECHST AG, nowadays SANOFI-AVENTIS.
Subsequent outbreaks appeared on the African continent:
1.) SOUTH AFRICA: In Johannesburg 1,975
2.) KENYA: Nairobi 1.980, and in the cave of Kitum 1,987,
3.) RUSSIA: in Koltsovo in 1.998-98.
4.) DEMOCRATIC REPUBLIC OF CONGO: in Durba and Watsa, 1.998- 2,000.
5.) UGANDA: Kitaka Mine 2.007 and Forest of Maramagambo 2.008.
6.) NETHERLANDS AND THE UNITED STATES: 2 cases imported 2.008.
6.) ANGOLA: in the province of Uije 2.004-2.005
7.) UGANDA: in Kabale 2.012.
8.) UGANDA: in 2.014.
The family of the FILOVIRUS consists of 8 different species: EBOLA VIRUS, LLOVIU VIRUS, MARBURG VIRUS, RESTON VIRUS, SUDAN VIRUS, TAI VIRUS, BUNDIBUGYO VIRUS and RAVN VIRUS, of these RAVN VIRUS is considered a close relative of MARBURG VIRUS AND ALSO It causes HEMORRHAGIC FEVER disease (MVD) by MARBURG VIRUS.
This virus is considered by the CDC (Centers for Disease Control and Prevention) a pathogen CATEGORY A of BIOTERRORISM agent in whose list are: TULAREMIA, ANTRAX, SMALLPOX, BOTULINUM TOXIN, BUBONIC PLAGUE and HEMORRAGIC VIRAL FEVERS which includes members of the family FILOVIRIDAE (Marburg and Ebola) and the family ARENAVIRIDAE (Lassa virus and Machupo Virus).
The TRANSMISSION of MARBURG VIRUS initially as I told you was the contact of HUMANS with tissues of AFRICAN GREEN MONKEYS infected in 1.967; Later it was verified that the BATS of the African FRUIT (Rousettus aegyptiacus) IS CARRIER OF THE MARBURG VIRUS(MARV).
There were outbreaks in Miners in the KITAKA CAVES in UGANDA, TOURISTS that visited the PHYTON cave in the same country, and other CAVES of Africa: KITUM CAVE in KENYA. In 2008, a DUTCH tourist became ill with the MARBURG VIRUS after visiting the PHYTON cave in UGANDA; it is believed that the feces pulverized in the environment were the mechanism of contagion. Later it was demonstrated that this BAT is the NATURAL RESERVOIR of the MARBUG VIRUS, and also of the EBOLA VIRUS.
It is also suspected that primates may also be a source of infection but have not been shown to be NATURAL RESERVOIRS.
HUMAN-HUMAN transmission was also demonstrated through direct contact with blood or other body fluids such as SALIVA, SWEAT, FECES, URINE, TEARS, AND BREAST MILK from infected patients. Even the virus has been found in TEARS, SEMEN, and HEPATIC BIOPSY weeks to months after infection, this being important for the monitoring of convalescent patients. CONTACT with infected patients and HANDLING of cadavers is also a source of infection and therefore there are rigid protocols for manipulation of patients.
SYMPTOMS OF MARBURG VIRUS DISEASE (MVD):
==========================================
The DISEASE is called HEMORRHAGIC FEVER (HF) BY MARBURG VIRUS (MARV), but it should be noted that the RAVN VIRUS in addition to the MARBURG VIRUS is also causal agent of the same.
The INCUBATION Period: has an average of 5-9 days but can reach 21 days. Then a phase of GENERALIZATION is presented: (day 1-5) with HEADACHES, CHILLS, NAUSEAS, VOMITING, FATIGUE, DIARRHEA, CUTANEOUS ERUPTION, ABDOMINAL PAIN, MALAISE. Then there is an EARLY ORGANIC phase, (day 5-13) which includes DYSPNEA, EDEMA CONJUNCTIVAL INJECTION, EXANTHEMA, CNS SYMPTOMS: ENCEPHALITIS, CONFUSION, DELIRIUM, APATHY, AGRESSION, then there are HEMORRHAGIC symptoms that produce hypovolemia, FECES WITH BLOOD, ECCHYMOSES, HEMATEMSESIS, HEMORRHAGES OF MUCOSAS AND VISCERAL, LOSS OF BLOOD IN THE VENIPUNCTURE SITES, in this phase the patient takes two routes: recovery or death.
The POPULAR belief is that the patient dies for HEMORRHAGES and this is not true. The patient goes to the LATE ORGANIC stage (day 13 - 21) where he enters CONVALESCENCE or continuous deteriorating state presenting PERSISTENT FEVER, CONVULSIONS, COMA, DISSEMINATED INTRAVASCULAR COAGULATION (CID), METABOLIC DISTURB, SHOCK AND DEATH that usually occurs between the day 8-16.
The MORTALITY for MARBUG VIRUS oscillates between 80-90%, for some scientists it is the second most LETAL virus for HUMANITY after EBOLA VIRUS.
TREATMENT
==========
There is no specific treatment against Marburg Virus disease or VACCINES available. Treatment is based on maintaining electrolytes, fluids, blood replacement, coagulation factors, blood pressure ... and pray a lot for the patient to save his life.! so that..
The famous character BATMAN lives in a CAVE WHERE ALSO LIVES BATS hence his name, and goes out at night to fight against the BAD PEOPLE of GOTIC city (GOTHAM), hopefully never get infected with MARBURG VIRUS, as it would be the end of the movie...
MORAL or LESSON: In the case of MARBURG VIRUS, the man once again invaded the natural habitat of the NATURAL RESERVOIR, the BATS on the CAVES, in search of precious metals ,,, and encountered disease and DEATH.. And you, if you are a tourist and you are going to VISIT A CAVE look at the ROOF and avoid the BATS. !
Greetings to all.
Dr. José Lapenta
18.) Biohazard
The Chilling True Story of the Largest Covert Biological Weapons Program in the World -- Told from Inside by the Man Who Ran It
========================================================================
By KEN ALIBEK with STEPHEN HANDELMAN
Source:http://www.nytimes.com/books/99/06/20/reviews/990620.20taubmat.html
Tularemia is a highly infectious disease that produces headaches, nausea and high fevers. It can be lethal if untreated. Tularemia is also hard to extinguish, making it attractive to anyone trying to produce biological weapons.
That's just what Ken Alibek was doing for the Soviet Union in 1983 when he found himself standing in a puddle of tularemia bacteria that had accidentally spilled onto the floor of a secret weapons lab. There was enough tularemia in the small, milky brown pool to infect everyone in the Soviet Union. Within hours Alibek was too sick to move. Only megadoses of tetracycline, hastily obtained from a friend, prevented the disease from disabling if not killing him.
That is one of many harrowing moments that Alibek describes in this absorbing account of the Soviet Union's demonic effort to make biological weapons. The program was one of the best-kept Russian secrets of the cold war, and Alibek was one of its central architects. He reports that at its high point in the late 1980's, when Mikhail Gorbachev was the Soviet leader, the program consumed close to $1 billion a year and employed more than 60,000 people at dozens of clandestine sites. Needless to say, it was not an activity that Gorbachev advertised as he tried to improve relations with the West.
Though the Russian effort is now believed to be largely abandoned, biological weapons remain a threat, perhaps even a greater one today because they can be made relatively easily and inexpensively by terrorist groups and leaders like Saddam Hussein.
Alibek, born Kanatjan Alibekov, defected to the United States in 1992 and changed his name. By then he had quit the weapons project in disgust, but for nearly all his career as a Soviet scientist he excelled at the grim business of cultivating biological agents and adapting them for use in missiles and bombs. For many years he was deputy director of Biopreparat, an ostensibly civilian agency that was actually involved in advanced research into biological weapons. Alibek provided American officials with their first full description of the Soviet effort when he defected.
In ''Biohazard'' he performs the same service for readers, with a strong writing assist from Stephen Handelman, who was a Moscow correspondent for The Toronto Star. The book works best as a richly descriptive report on the Soviet program and Alibek's role in it. It is less successful as a portrait of Alibek and his transition from germ warfare acolyte to apostate.
The story is sobering. With no limit to the resources it was prepared to invest in unconventional weapons research, the Soviet Union developed an extensive arsenal of deadly pathogens, including anthrax, smallpox, plague, brucellosis and tularemia. Tons of these bacteria and viruses were churned out at production centers, often in vaccine-resistant strains that could be effectively dispersed in liquid, powder or aerosol form. Moscow even tried to manipulate the AIDS virus so it could be used as a weapon. The disease's long incubation period made it unsuitable.
For Alibek and his colleagues, the grotesque work was just another day at the office. He recalls a meeting in 1988 at Soviet Army headquarters in Moscow, where he was instructed to arm long-range missiles with deadly germs. ''I made a few quick calculations on my note pad,'' he says. ''At least 400 kilograms of anthrax, prepared in dry form for use as an aerosol, would be required for 10 warheads.'' Martha Stewart couldn't have put it more innocuously.
The Kremlin went ahead with such work even though it had signed the 1972 Biological Weapons Convention, which banned the development, production and stockpiling of biological agents for offensive military purposes. Just a year after signing the accord, the Soviet Government secretly initiated an effort to modernize its biological weapons and to invent new ones. The United States, for its part, maintained a robust biological warfare program until 1969, when President Richard Nixon renounced the use of such weapons and restricted research to defensive measures like immunization.
Alibek was drawn into the Soviet campaign in 1975, deflected from a conventional career as a military physician by the allure of highly classified research, the prospect of rapid advancement and the mistaken belief that the Soviet Union had no choice but to keep pace with the United States in germ warfare technology. He was a Kazakh native eager to prove himself to his Russian superiors. With a knack for epidemiology and laboratory research, he was soon building what he describes matter-of-factly as ''the world's most efficient assembly line for the mass production of weaponized anthrax.''
Alibek has a fine eye for the cold-blooded customs of the Soviet state, including coercion and deception. He never told his supervisors about his frightening bout with tularemia, fearing it would cost his job. A few years earlier, while a student at the Tomsk Medical Institute in Siberia, he had surmised from medical records that Soviet forces had used the same disease as a weapon against German troops outside Stalingrad in 1942. His professor, a colonel, icily told Alibek, ''You have gone beyond your assignment,'' and advised him never to speak of the matter again.
Though Alibek struggles to explain his enthusiasm for biological weapons work, he seems reluctant to probe beyond surface emotions. He stops the narrative periodically for moments of introspection like this: ''I still shuddered occasionally when I looked at the bacteria multiplying in our fermenters and considered that they could end the lives of millions of people. But the secret culture of our labs had changed my outlook. My parents would not have recognized the man I had become.'' Unhappily, these tantalizing passages are but brief digressions, leaving one to puzzle over just why Alibek turned against the system.
The Russian program was theoretically dismantled in recent years at the order of President Boris N. Yeltsin, but Ken Alibek makes clear there may still be active remnants. Given the unblinking support he and thousands of others gave to the effort, that would not be surprising.
===========================================================================
19.) Ebola and Marburg virus vaccines.
===========================================================================
Virus Genes. 2017 Aug;53(4):501-515. doi: 10.1007/s11262-017-1455-x. Epub 2017 Apr 26.
Reynolds P1, Marzi A2.
Author information
1
Laboratory of Virology, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Hamilton, MT, USA.
2
Laboratory of Virology, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Hamilton, MT, USA. marzia@niaid.nih.gov.
Abstract
The filoviruses, Ebola virus (EBOV), and Marburg virus (MARV), are among the most pathogenic viruses known to man and the causative agents of viral hemorrhagic fever outbreaks in Africa with case fatality rates of up to 90%. Nearly 30,000 infections were observed in the latest EBOV epidemic in West Africa; previous outbreaks were much smaller, typically only affecting less than a few hundred people. Compared to other diseases such as AIDS or Malaria with millions of cases annually, filovirus hemorrhagic fever (FHF) is one of the neglected infectious diseases. There are no licensed vaccines or therapeutics available to treat EBOV and MARV infections; therefore, these pathogens can only be handled in maximum containment laboratories and are classified as select agents. Under these limitations, a very few laboratories worldwide conducted basic research and countermeasure development for EBOV and MARV since their respective discoveries in 1967 (MARV) and 1976 (EBOV). In this review, we discuss several vaccine platforms against EBOV and MARV, which have been assessed for their protective efficacy in animal models of FHF. The focus is on the most promising approaches, which were accelerated in clinical development (phase I-III trials) during the EBOV epidemic in West Africa.
======================================================================== ==================
Hello friends of the DERMAGIC EXPRESS network today brings you a very interesting topic, it is THE MARBURG VIRUS (MARV), THE NIGHT OF BATS , virus today considered ONE OF THE MOST LETHAL VIRUSES for HUMANITY, after the EBOLA VIRUS, although both belong to the same family of FILOVIRUS.
But perhaps the most AMAZING I found in this review is that this virus in the years of the COLD WAR in the SOVIET UNION was one of the many biological agents that I try to be used as a biological weapon, to be placed in the nuclear warheads of the Russian missiles. This fact was denounced by the Russian scientist Ken Alibek, who worked in one of the laboratories in the years 1.983-1.988 for such purposes and desert to the United States of America later and revealed these state secrets.
Another detail to consider if you read previous reviews, is that the habitat where humans live is NOT SO INOFENSIVE, we are surrounded by TICKS (Lyme disease, Powassan, Heartland, Crimea-Congo fever and others) INSECTS (Zika, Dengue , Chikungunya, Nile Fever, Japanese Encephalitis, jamestown canyon virus and others), RATS, MICE (Hantavirus, Bubonic Plague, Leptospirosis, Salmonella and others) virus TRANSMITTERS of diseases that are highly LETHAL or inoculate you with a disease that may leave you sick lifelong . And today I bring you also these, THE BATS, harmless FLYERS MAMMALS that live in caves, and nocturnal activity, NATURAL RESERVOIRS OF THE VIRUSES OF MARBURG and EBOLA considered today the 2 MOST LETHAL viruses for humanity with mortality greater than 80%. After the contagion.
THE MARBURG VIRUS (MARV) belongs to the group: RNA VIRUS, order: MONONEGAVIRALES, family: FILOVIRIDAE, genus: MARBURGVIRUS, species: MARGBURG MARBURGVIRUS.
MARBURG VIRUS appeared for the first time in 1.967 in a LABORATORY in the localities of MARBURG and FRANKFURT in Germany and in Belgrade, Yugoslavia (today Serbia), when workers were infected by an unknown pathogen that provoked HEMORRHAGIC manifestations and high lethality. The causal agent was isolated and identified by scientists from the University of MARBURG, Germany hence its name. The virus carriers were infected AFRICAN GREEN MONKEYS (Chlorocebus aethiops) that had been imported to be used in the manufacture of vaccines in the German HOECHST AG, nowadays SANOFI-AVENTIS.
Subsequent outbreaks appeared on the African continent:
1.) SOUTH AFRICA: In Johannesburg 1,975
2.) KENYA: Nairobi 1.980, and in the cave of Kitum 1,987,
3.) RUSSIA: in Koltsovo in 1.998-98.
4.) DEMOCRATIC REPUBLIC OF CONGO: in Durba and Watsa, 1.998- 2,000.
5.) UGANDA: Kitaka Mine 2.007 and Forest of Maramagambo 2.008.
6.) NETHERLANDS AND THE UNITED STATES: 2 cases imported 2.008.
6.) ANGOLA: in the province of Uije 2.004-2.005
7.) UGANDA: in Kabale 2.012.
8.) UGANDA: in 2.014.
The family of the FILOVIRUS consists of 8 different species: EBOLA VIRUS, LLOVIU VIRUS, MARBURG VIRUS, RESTON VIRUS, SUDAN VIRUS, TAI VIRUS, BUNDIBUGYO VIRUS and RAVN VIRUS, of these RAVN VIRUS is considered a close relative of MARBURG VIRUS AND ALSO It causes HEMORRHAGIC FEVER disease (MVD) by MARBURG VIRUS.
This virus is considered by the CDC (Centers for Disease Control and Prevention) a pathogen CATEGORY A of BIOTERRORISM agent in whose list are: TULAREMIA, ANTRAX, SMALLPOX, BOTULINUM TOXIN, BUBONIC PLAGUE and HEMORRAGIC VIRAL FEVERS which includes members of the family FILOVIRIDAE (Marburg and Ebola) and the family ARENAVIRIDAE (Lassa virus and Machupo Virus).
The TRANSMISSION of MARBURG VIRUS initially as I told you was the contact of HUMANS with tissues of AFRICAN GREEN MONKEYS infected in 1.967; Later it was verified that the BATS of the African FRUIT (Rousettus aegyptiacus) IS CARRIER OF THE MARBURG VIRUS(MARV).
There were outbreaks in Miners in the KITAKA CAVES in UGANDA, TOURISTS that visited the PHYTON cave in the same country, and other CAVES of Africa: KITUM CAVE in KENYA. In 2008, a DUTCH tourist became ill with the MARBURG VIRUS after visiting the PHYTON cave in UGANDA; it is believed that the feces pulverized in the environment were the mechanism of contagion. Later it was demonstrated that this BAT is the NATURAL RESERVOIR of the MARBUG VIRUS, and also of the EBOLA VIRUS.
It is also suspected that primates may also be a source of infection but have not been shown to be NATURAL RESERVOIRS.
HUMAN-HUMAN transmission was also demonstrated through direct contact with blood or other body fluids such as SALIVA, SWEAT, FECES, URINE, TEARS, AND BREAST MILK from infected patients. Even the virus has been found in TEARS, SEMEN, and HEPATIC BIOPSY weeks to months after infection, this being important for the monitoring of convalescent patients. CONTACT with infected patients and HANDLING of cadavers is also a source of infection and therefore there are rigid protocols for manipulation of patients.
SYMPTOMS OF MARBURG VIRUS DISEASE (MVD):
==========================================
The DISEASE is called HEMORRHAGIC FEVER (HF) BY MARBURG VIRUS (MARV), but it should be noted that the RAVN VIRUS in addition to the MARBURG VIRUS is also causal agent of the same.
The INCUBATION Period: has an average of 5-9 days but can reach 21 days. Then a phase of GENERALIZATION is presented: (day 1-5) with HEADACHES, CHILLS, NAUSEAS, VOMITING, FATIGUE, DIARRHEA, CUTANEOUS ERUPTION, ABDOMINAL PAIN, MALAISE. Then there is an EARLY ORGANIC phase, (day 5-13) which includes DYSPNEA, EDEMA CONJUNCTIVAL INJECTION, EXANTHEMA, CNS SYMPTOMS: ENCEPHALITIS, CONFUSION, DELIRIUM, APATHY, AGRESSION, then there are HEMORRHAGIC symptoms that produce hypovolemia, FECES WITH BLOOD, ECCHYMOSES, HEMATEMSESIS, HEMORRHAGES OF MUCOSAS AND VISCERAL, LOSS OF BLOOD IN THE VENIPUNCTURE SITES, in this phase the patient takes two routes: recovery or death.
The POPULAR belief is that the patient dies for HEMORRHAGES and this is not true. The patient goes to the LATE ORGANIC stage (day 13 - 21) where he enters CONVALESCENCE or continuous deteriorating state presenting PERSISTENT FEVER, CONVULSIONS, COMA, DISSEMINATED INTRAVASCULAR COAGULATION (CID), METABOLIC DISTURB, SHOCK AND DEATH that usually occurs between the day 8-16.
The MORTALITY for MARBUG VIRUS oscillates between 80-90%, for some scientists it is the second most LETAL virus for HUMANITY after EBOLA VIRUS.
TREATMENT
==========
There is no specific treatment against Marburg Virus disease or VACCINES available. Treatment is based on maintaining electrolytes, fluids, blood replacement, coagulation factors, blood pressure ... and pray a lot for the patient to save his life.! so that..
The famous character BATMAN lives in a CAVE WHERE ALSO LIVES BATS hence his name, and goes out at night to fight against the BAD PEOPLE of GOTIC city (GOTHAM), hopefully never get infected with MARBURG VIRUS, as it would be the end of the movie...
MORAL or LESSON: In the case of MARBURG VIRUS, the man once again invaded the natural habitat of the NATURAL RESERVOIR, the BATS on the CAVES, in search of precious metals ,,, and encountered disease and DEATH.. And you, if you are a tourist and you are going to VISIT A CAVE look at the ROOF and avoid the BATS. !
Greetings to all.
Dr. José Lapenta
=======================================================================
REFERENCIAS BIBLIOGRAFICAS/ BIBLIOGRAPHICAL REFERENCES
=======================================================================
1.) Filovirus Research: How it Began.
2.) Forty-five years of Marburg virus research.
3.) Marburg haemorrhagic fever in returning travellers: an overview aimed at clinicians.
4.) Imported case of Marburg hemorrhagic fever - Colorado, 2008.
5.) Seasonal pulses of Marburg virus circulation in juvenile Rousettus aegyptiacus bats coincide with periods of increased risk of human infection.
6.) Repeated outbreaks of viral hemorrhagic fevers in Uganda.
7.) Oral shedding of Marburg virus in experimentally infected Egyptian fruit bats (Rousettus aegyptiacus).
8.) Mapping the zoonotic niche of Marburg virus disease in Africa.
9.) Is Marburg virus enzootic in Gabon?
10.) Marburg virus infection detected in a common African bat.
11.) Studies of reservoir hosts for Marburg virus.
12.) Marburgvirus Resurgence in Kitaka Mine Bat Population after Extermination Attempts, Uganda
13.) Marburgvirus genomics and association with a large hemorrhagic fever outbreak in Angola.
14.) Isolation of genetically diverse Marburg viruses from Egyptian fruit bats.
15.) Filoviruses and bats.
16.) Outbreak of Marburg hemorrhagic fever among miners in Kamwenge and Ibanda Districts, Uganda, 2007.
17.) Guide to the Correct Use of Filoviral Nomenclature.
18.) Biohazard
The Chilling True Story of the Largest Covert Biological Weapons Program in the World -- Told from Inside by the Man Who Ran It
19.) Ebola and Marburg virus vaccines.
.===============================================================
===============================================================
1.) Filovirus Research: How it Began.
===============================================================
Curr Top Microbiol Immunol. 2017 Aug 2. doi: 10.1007/82_2017_8. [Epub ahead of print]
Slenczka W1.
Author information
1
Institute of Virology, Philipps University of Marburg, Am Weinberg 19, 35037, Marburg, Germany. slenczka-marburg@t-online.de.
Abstract
The first reported filovirus outbreak occurred in August 1967, when laboratory workers in Marburg and Frankfurt, Germany, and Belgrade, Yugoslavia (now Serbia) became infected with an unknown highly pathogenic agent. The disease was characterized by high fever, malaise, rash, hemorrhagic and tetanic manifestations, and high lethality, amounting to 25%. The disease was introduced to Europe by grivets (Chlorocebus aethiops), which were used for biomedical research and vaccine production. The causative agent, Marburg virus, was isolated and identified by scientists of the University of Marburg, Germany in cooperation with specialists for viral electron microscopy at the Bernhard Nocht Institute in Hamburg, Germany. In this chapter, Dr. Slenczka, who was involved in the first isolation of Marburg virus in 1967, describes the desperate hunt of the causative agent of this first filovirus disease outbreak in the center of Europe, its successful isolation, the likely route of transmission from a monkey trading station to vaccine production facilities in Germany and Yugoslavia, and the consequences of this outbreak, including a shortage in the production of poliomyelitis vaccine In addition, this chapter provides insight into some of the peculiarities of filovirus infection, such as sexual virus transmission several months after recovery and the role of Ca2+-loss in Marburg virus pathogenesis, which were already observed during this first well-documented Marburg virus disease outbreak.
=========================================================================
2.) Forty-five years of Marburg virus research.
========================================================================
Viruses. 2012 Oct 1;4(10):1878-927. doi: 10.3390/v4101878.
Brauburger K1, Hume AJ, Mühlberger E, Olejnik J.
Author information
1
Department of Microbiology, School of Medicine and National Emerging Infectious Diseases Laboratories Institute, Boston University, Boston, MA 02118, USA. brauburk@bu.edu
Abstract
In 1967, the first reported filovirus hemorrhagic fever outbreak took place in Germany and the former Yugoslavia. The causative agent that was identified during this outbreak, Marburg virus, is one of the most deadly human pathogens. This article provides a comprehensive overview of our current knowledge about Marburg virus disease ranging from ecology to pathogenesis and molecular biology.
=========================================================================
3.) Marburg haemorrhagic fever in returning travellers: an overview aimed at clinicians.
========================================================================
Clin Microbiol Infect. 2015 Jun 22. pii: S1198-743X(15)00538-8. doi: 10.1111/1469-0691.12673. [Epub ahead of print]
Bauer MP1, Timen A2, Vossen AC3, van Dissel JT4.
Author information
1
Department of Infectious Diseases, Leiden University Medical Centre, Leiden, The Netherlands.
2
National Institute for Public Health and the Environment, Bilthoven, The Netherlands.
3
Department Medical Microbiology, Leiden University Medical Centre, Leiden, The Netherlands.
4
Department of Infectious Diseases, Leiden University Medical Centre, Leiden, The Netherlands. Electronic address: e.j.t.van_dissel@lumc.nl.
Abstract
Marburg virus haemorrhagic fever (MARV HF) is a dramatic disease that can occur in a traveller returning from an area where the virus is endemic. In this article, we provide an overview of MARV HF as an imported infection with an emphasis on clinical aspects. Although late features such as rash, signs of haemorrhagic diathesis and liver necrosis may point to the diagnosis, the initial clinical picture is non-specific. If in this early phase the patient's epidemiological exposure history is compatible with MARV HF, the patient should be isolated and managed according to viral haemorrhagic fever protocol and RT-PCR should be performed on the patient's blood as soon as possible to rule out MARV HF (or other possible viral haemorrhagic fevers). In severe cases, direct electron microscopy of blood in specialized centres (e.g. Bernhard-Nocht Institute in Hamburg, Germany) may be considered if the result of the RT-PCR is not readily available. Adequate diagnostics and empirical treatment for other acute life-threatening illnesses should not be withheld while test results are awaited, but all management and diagnostics should be weighed against the risks of nosocomial transmission.
=========================================================================
4.) Imported case of Marburg hemorrhagic fever - Colorado, 2008.
========================================================================
Centers for Disease Control and Prevention (CDC).
Abstract
Marburg hemorrhagic fever (MHF) is a rare, viral hemorrhagic fever (VHF); the causative agent is an RNA virus in the family Filoviridae, and growing evidence demonstrates that fruit bats are the natural reservoir of Marburg virus (MARV). On January 9, 2008, an infectious disease physician notified the Colorado Department of Public Health and Environment (CDPHE) of a case of unexplained febrile illness requiring hospitalization in a woman who had returned from travel in Uganda. Testing of early convalescent serum demonstrated no evidence of infection with agents that cause tropical febrile illnesses, including VHF. Six months later, in July 2008, the patient requested repeat testing after she learned of the death from MHF of a Dutch tourist who had visited the same bat-roosting cave as the patient, the Python Cave in Queen Elizabeth National Park, Uganda. The convalescent serologic testing revealed evidence of prior infection with MARV, and MARV RNA was detected in the archived early convalescent serum. A public health investigation did not identify illness consistent with secondary MHF transmission among her contacts, and no serologic evidence of infection was detected among the six tested of her eight tour companions. The patient might have acquired MARV infection through exposure to bat secretions or excretions while visiting the Python Cave. Travelers should be aware of the risk for acquiring MHF in caves or mines inhabited by bats in endemic areas in sub-Saharan Africa. Health-care providers should consider VHF among travelers returning from endemic areas who experience unexplained febrile illness.
=========================================================================
5.) Seasonal pulses of Marburg virus circulation in juvenile Rousettus aegyptiacus bats coincide with periods of increased risk of human infection.
=========================================================================
PLoS Pathog. 2012;8(10):e1002877. doi: 10.1371/journal.ppat.1002877. Epub 2012 Oct 4.
Amman BR1, Carroll SA, Reed ZD, Sealy TK, Balinandi S, Swanepoel R, Kemp A, Erickson BR, Comer JA, Campbell S, Cannon DL, Khristova ML, Atimnedi P, Paddock CD, Crockett RJ, Flietstra TD, Warfield KL, Unfer R, Katongole-Mbidde E, Downing R, Tappero JW, Zaki SR, Rollin PE, Ksiazek TG, Nichol ST, Towner JS.
Author information
1
Viral Special Pathogens Branch, Centers for Disease Control and Prevention, Atlanta, Georgia, United States of America.
Abstract
Marburg virus (family Filoviridae) causes sporadic outbreaks of severe hemorrhagic disease in sub-Saharan Africa. Bats have been implicated as likely natural reservoir hosts based most recently on an investigation of cases among miners infected in 2007 at the Kitaka mine, Uganda, which contained a large population of Marburg virus-infected Rousettus aegyptiacus fruit bats. Described here is an ecologic investigation of Python Cave, Uganda, where an American and a Dutch tourist acquired Marburg virus infection in December 2007 and July 2008. More than 40,000 R. aegyptiacus were found in the cave and were the sole bat species present. Between August 2008 and November 2009, 1,622 bats were captured and tested for Marburg virus. Q-RT-PCR analysis of bat liver/spleen tissues indicated ~2.5% of the bats were actively infected, seven of which yielded Marburg virus isolates. Moreover, Q-RT-PCR-positive lung, kidney, colon and reproductive tissues were found, consistent with potential for oral, urine, fecal or sexual transmission. The combined data for R. aegyptiacus tested from Python Cave and Kitaka mine indicate low level horizontal transmission throughout the year. However, Q-RT-PCR data show distinct pulses of virus infection in older juvenile bats (~six months of age) that temporarily coincide with the peak twice-yearly birthing seasons. Retrospective analysis of historical human infections suspected to have been the result of discrete spillover events directly from nature found 83% (54/65) events occurred during these seasonal pulses in virus circulation, perhaps demonstrating periods of increased risk of human infection. The discovery of two tags at Python Cave from bats marked at Kitaka mine, together with the close genetic linkages evident between viruses detected in geographically distant locations, are consistent with R. aegyptiacus bats existing as a large meta-population with associated virus circulation over broad geographic ranges. These findings provide a basis for developing Marburg hemorrhagic fever risk reduction strategies.
========================================================================
6.) Repeated outbreaks of viral hemorrhagic fevers in Uganda.
========================================================================
Mbonye A1, Wamala J, Winyi-Kaboyo, Tugumizemo V, Aceng J, Makumbi I.
Author information
1
Ministry of Health Head Quarters, P.O Box 7272 Kampala, Uganda. vpadmn@infocom.co.ug
Abstract
BACKGROUND:
Since the year 2000, Uganda has experienced repeated outbreaks of viral hemorrhagic fevers (VHF). Ebola VHF outbreak occurred in the districts of Gulu in 2000, Bundibugyo, 2007, Luwero, 2011, Kibaale in July 2012, Luwero in November 2012. Marburg VHF was earlier reported in Ibanda in 2007. More recently in 2012, two outbreaks of Marburg VHF have occurred in Ibanda and Kabale districts.
OBJECTIVE:
To present the epidemiological picture of the Marburg VHF recently reported in Ibanda and Kabale districts and propose research questions to generate evidence to mitigate future epidemics.
METHODS:
A case definition for a VHF was developed. A frequency distribution of symptoms of confirmed and probable cases was done. Descriptive analyses of reported cases using simple percentages, percent distributions and computation of means was performed.
RESULTS:
The Marburg epidemic was reported in early September and by November 2012, a cumulative of 14 cases (9 confirmed and 5 probable) including 7 deaths had been registered, giving a case fatality rate (CFR) of 50%. A total of 202 contacts had been listed; out of which 193 had completed the 21-day follow-up period. The index case was a 33-year old male, a teacher at Nyakatukura Secondary School in Ibanda district. He travelled to Ibanda from Kabale, his home district on 31st August 2012, reportedly healthy. He fell sick on 3rd September 2012 with complaints of fever, headache, loss of appetite and general body weakness. Overall, the dominant symptoms for all cases were fever, vomiting, loss of appetite, headache, abdominal pain, fatigue, diarrhea, and the least in occurrence was bleeding which accounted for 35.5% of all the cases.
CONCLUSION:
The source of infection for all the five Ebola Hemorrhagic fever outbreaks in Uganda and the recent Marburg VHF outbreak in Ibanda and Kabale is not known. Currently there is suspicion that there could be an animal reservoir of the Ebola and Marburg viruses from where occasional spillage into the human population occurs resulting in disease outbreaks. This and other hypotheses require further investigation.
========================================================================
7.) Oral shedding of Marburg virus in experimentally infected Egyptian fruit bats (Rousettus aegyptiacus).
========================================================================
J Wildl Dis. 2015 Jan;51(1):113-24. doi: 10.7589/2014-08-198.
Amman BR1, Jones ME, Sealy TK, Uebelhoer LS, Schuh AJ, Bird BH, Coleman-McCray JD, Martin BE, Nichol ST, Towner JS.
Author information
1
1 Centers for Disease Control and Prevention, Viral Special Pathogens Branch, 1600 Clifton Rd. NE, Atlanta, Georgia 30333, USA.
Abstract
Marburg virus (Marburg marburgvirus; MARV) causes sporadic outbreaks of Marburg hemorrhagic fever (MHF) in Africa. The Egyptian fruit bat (Rousettus aegyptiacus) has been identified as a natural reservoir based most-recently on the repeated isolation of MARV directly from bats caught at two locations in southwestern Uganda where miners and tourists separately contracted MHF from 2007-08. Despite learning much about the ecology of MARV through extensive field investigations, there remained unanswered questions such as determining the primary routes of virus shedding and the severity of disease, if any, caused by MARV in infected bats. To answer these questions and others, we experimentally infected captive-bred R. aegyptiacus with MARV under high (biosafety level 4) containment. These experiments have shown infection profiles consistent with R. aegyptiacus being a bona fide natural reservoir host for MARV and demonstrated routes of viral shedding capable of infecting humans and other animals.
========================================================================
8.) Mapping the zoonotic niche of Marburg virus disease in Africa.
=======================================================================
Trans R Soc Trop Med Hyg. 2015 Jun;109(6):366-78. doi: 10.1093/trstmh/trv024. Epub 2015 Mar 27.
Pigott DM1, Golding N2, Mylne A2, Huang Z2, Weiss DJ2, Brady OJ2, Kraemer MU2, Hay SI3.
Author information
1
Spatial Ecology & Epidemiology Group, Department of Zoology, University of Oxford, Oxford, UK david.pigott@zoo.ox.ac.uk.
2
Spatial Ecology & Epidemiology Group, Department of Zoology, University of Oxford, Oxford, UK.
3
Spatial Ecology & Epidemiology Group, Department of Zoology, University of Oxford, Oxford, UK Fogarty International Center, National Institutes of Health, Bethesda, Maryland, USA.
Abstract
BACKGROUND:
Marburg virus disease (MVD) describes a viral haemorrhagic fever responsible for a number of outbreaks across eastern and southern Africa. It is a zoonotic disease, with the Egyptian rousette (Rousettus aegyptiacus) identified as a reservoir host. Infection is suspected to result from contact between this reservoir and human populations, with occasional secondary human-to-human transmission.
METHODS:
Index cases of previous human outbreaks were identified and reports of infection in animals recorded. These data were modelled within a species distribution modelling framework in order to generate a probabilistic surface of zoonotic transmission potential of MVD across sub-Saharan Africa.
RESULTS:
Areas suitable for zoonotic transmission of MVD are predicted in 27 countries inhabited by 105 million people. Regions are suggested for exploratory surveys to better characterise the geographical distribution of the disease, as well as for directing efforts to communicate the risk of practices enhancing zoonotic contact.
CONCLUSIONS:
These maps can inform future contingency and preparedness strategies for MVD control, especially where secondary transmission is a risk. Coupling this risk map with patient travel histories could be used to guide the differential diagnosis of highly transmissible pathogens, enabling more rapid response to outbreaks of haemorrhagic fever.
========================================================================
9.) Is Marburg virus enzootic in Gabon?
========================================================================
J Infect Dis. 2011 Nov;204 Suppl 3:S800-3. doi: 10.1093/infdis/jir358.
Maganga GD1, Bourgarel M, Ella GE, Drexler JF, Gonzalez JP, Drosten C, Leroy EM.
Author information
1
Centre International de Recherches Médicales de Franceville, Gabon.
Abstract
Marburg virus (MARV) nucleic acid was detected in Rousettus aegyptiacus bats in 2005 and 2006 in the midwest and southeast of Gabon. In this study we used MARV-specific real-time reverse-transcription polymerase chain reaction (RT-PCR) and MARV-specific nested RT-PCR assay to screen 1257 bats caught during July 2009, December 2009, and June 2010 in 3 caves situated in northern Gabon. Nine specimens tested positive by the real-time assay, with cycle threshold values ranging from 35 to 39, of which only 1 R. aegyptiacus specimen collected in 2009 was positive in the nested VP35 RT-PCR assay. Together with MARV-positive bats in the south and west found in 2005 and 2006, confirmation of phylogenetically closely related MARV-positive bats 5 years later and in northern Gabon suggests that MARV is now enzootic in Gabon and emphasizes the importance of long-term monitoring of bat populations and human-bat interfaces.
========================================================================
10.) Marburg virus infection detected in a common African bat.
========================================================================
PLoS One. 2007 Aug 22;2(8):e764.
Towner JS1, Pourrut X, Albariño CG, Nkogue CN, Bird BH, Grard G, Ksiazek TG, Gonzalez JP, Nichol ST, Leroy EM.
Author information
1
Centers for Disease Control and Prevention, Special Pathogens Branch, Atlanta, Georgia, United States of America.
Abstract
Marburg and Ebola viruses can cause large hemorrhagic fever (HF) outbreaks with high case fatality (80-90%) in human and great apes. Identification of the natural reservoir of these viruses is one of the most important topics in this field and a fundamental key to understanding their natural history. Despite the discovery of this virus family almost 40 years ago, the search for the natural reservoir of these lethal pathogens remains an enigma despite numerous ecological studies. Here, we report the discovery of Marburg virus in a common species of fruit bat (Rousettus aegyptiacus) in Gabon as shown by finding virus-specific RNA and IgG antibody in individual bats. These Marburg virus positive bats represent the first naturally infected non-primate animals identified. Furthermore, this is the first report of Marburg virus being present in this area of Africa, thus extending the known range of the virus. These data imply that more areas are at risk for MHF outbreaks than previously realized and correspond well with a recently published report in which three species of fruit bats were demonstrated to be likely reservoirs for Ebola virus.
========================================================================
11.) Studies of reservoir hosts for Marburg virus.
========================================================================
Emerg Infect Dis. 2007 Dec;13(12):1847-51. doi: 10.3201/eid1312.071115.
Swanepoel R1, Smit SB, Rollin PE, Formenty P, Leman PA, Kemp A, Burt FJ, Grobbelaar AA, Croft J, Bausch DG, Zeller H, Leirs H, Braack LE, Libande ML, Zaki S, Nichol ST, Ksiazek TG, Paweska JT; International Scientific and Technical Committee for Marburg Hemorrhagic Fever Control in the Democratic Republic of Congo.
Author information
1
National Institute for Communicable Diseases, Sandringham, Republic of South Africa. bobs@nicd.ac.za
Abstract
To determine reservoir hosts for Marburg virus (MARV), we examined the fauna of a mine in northeastern Democratic Republic of the Congo. The mine was associated with a protracted outbreak of Marburg hemorrhagic fever during 1998-2000. We found MARV nucleic acid in 12 bats, comprising 3.0%-3.6% of 2 species of insectivorous bat and 1 species of fruit bat. We found antibody to the virus in the serum of 9.7% of 1 of the insectivorous species and in 20.5% of the fruit bat species, but attempts to isolate virus were unsuccessful
========================================================================
12.) Marburgvirus Resurgence in Kitaka Mine Bat Population after Extermination Attempts, Uganda
=======================================================================
Emerg Infect Dis. 2014 Oct; 20(10): 1761–1764. doi: 10.3201/eid2010.140696
Brian R. Amman, Luke Nyakarahuka, Anita K. McElroy, Kimberly A. Dodd, Tara K. Sealy, Amy J. Schuh, Trevor R. Shoemaker, Stephen Balinandi, Patrick Atimnedi, Winyi Kaboyo, Stuart T. Nichol, and Jonathan S. Townercorresponding author
To the Editor: Marburg virus (MARV) and Ravn virus (RAVV), collectively called marburgviruses, cause Marburg hemorrhagic fever (MHF) in humans. In July 2007, 4 cases of MHF (1 fatal) occurred in miners at Kitaka Mine in southern Uganda. Later, MHF occurred in 2 tourists who visited Python Cave, ≈50 km from Kitaka Mine. One of the tourists was from the United States (December 2007) and 1 was from the Netherlands (July 2008); 1 case was fatal (1,2,3). The cave and the mine each contained 40,000–100,000 Rousettus aegyptiacus bats (Egyptian fruit bats).
Longitudinal investigations of the outbreaks at both locations were initiated by the Viral Special Pathogens Branch of the Centers for Disease Control and Prevention (CDC, Atlanta, GA, USA, and Entebbe, Uganda) in collaboration with the Uganda Wildlife Authority (UWA) and the Uganda Virus Research Institute (UVRI). During these studies, genetically diverse MARVs and RAVVs were isolated directly from bat tissues, and infection levels of the 2 viruses were found to increase in juvenile bats on a predictable bi-annual basis (4,5). However, investigations at Kitaka Mine were stopped when the miners exterminated the bat colony by restricting egress from the cave with papyrus reed barriers and then entangling the bats in fishing nets draped over the exits. The trapping continued for weeks, and the entrances were then sealed with sticks and plastic. These depopulation efforts were documented by researchers from UVRI, the CDC, the National Institute of Communicable Diseases (Sandringham, South Africa), and UWA during site visits to Kitaka Mine (Technical Appendix Figure). In August 2008, thousands of dead bats were found piled in the forest, and by November 2008, there was no evidence of bats living in the mine; whether 100% extermination was achieved is unknown. CDC, UVRI, and UWA recommended against extermination, believing that any results would be temporary and that such efforts could exacerbate the problem if bat exclusion methods were not complete and permanent (6,7).
In October 2012, the most recent known marburgvirus outbreak was detected in Ibanda, a town in southwest Uganda. Ibanda is ≈20 km from the Kitaka Mine and is the urban center that serves smaller communities in the Kitaka area. This MHF outbreak was the largest in Ugandan history: 15 laboratory-confirmed cases occurred (8). In November 2012, an ecologic investigation of the greater Ibanda/Kitaka area was initiated. The investigation included interviews with local authorities to locate all known R. aegyptiacus colonies in the area. Although minor colonies of small insectivorous bats were found, the only identifiable colony of R. aegyptiacus bats was found inside the re-opened Kitaka Mine, albeit at much reduced size, perhaps 1%–5% of that found before depopulation efforts.
To determine whether the R. aegyptiacus bats that had repopulated Kitaka Mine were actively infected with marburgviruses, we tested 400 bats by using previously described methods (4,5). Viral RNA was extracted from ≈100 mg of liver and spleen tissue by using the MagMAX Total Nucleic Acid Isolation Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s recommended protocol. The Fisher exact test was conducted by using IBM SPSS Statistics, version 19.0 (IBM Corp., Armonk, NY, USA).
Of the 400 R. aegyptiacus bats collected, 53 (13.3%) were positive for marburgvirus RNA by quantitative reverse transcription PCR (32/233 [13.7%] adults and 21/167 [12.6%] juveniles; Technical Appendix Table); marburgvirus was isolated from tissue samples from 9 of the 400 bats. The overall level of active infection was significantly higher than that found in Kitaka Mine during 2007–2008 (5.1%) (5) (Fisher exact test, p<0.001) and in other studies in Uganda (Python Cave [2.5%]) and Gabon (4.8%) (4,9). The reason for the increase is not clear, but it may be related to the effects of the extermination and subsequent repopulation. Increases in disease prevalence in wildlife populations after culling are not unprecedented (6,7). We speculate that after the depopulation attempt, a pool of susceptible bats became established over time and was subjected to multiple marburgvirus introductions, as evidenced by the genetic diversity of viruses isolated from the bats (Figure). A pool of susceptible bats would have led to higher levels of active infection within the colony, thereby increasing the potential for virus spillover into the human population. A significant sex and age bias was not detected with respect to active infection during the breeding season (Fisher exact test, p>0.5 for both), and overall, the presence of virus-specific IgG among the bats was 16.5%, a finding consistent with that in previous studies (4,5).
Phylogeny of concatenated marburgvirus nucleoprotein (NP) and viral protein 35 (VP35) gene fragments as determined by using the maximum-likelihood method. Sequences from the NP (289–372 nt) and VP35 (203–213 nt) genes were amplified and ...
Phylogenetic analysis of viral RNA genome fragment sequences in this study showed high marburgvirus genetic diversity, including the presence of RAVVs and MARVs. Sequences for isolates from 3 bats were nearly identical to those of the MARV isolates obtained from patients in the 2012 Ibanda outbreak (8), suggesting that bats from Kitaka Mine were a likely source of the virus.
========================================================================
13.) Marburgvirus genomics and association with a large hemorrhagic fever outbreak in Angola.
=======================================================================
J Virol. 2006 Jul;80(13):6497-516.
Towner JS1, Khristova ML, Sealy TK, Vincent MJ, Erickson BR, Bawiec DA, Hartman AL, Comer JA, Zaki SR, Ströher U, Gomes da Silva F, del Castillo F, Rollin PE, Ksiazek TG, Nichol ST.
Author information
1
Special Pathogens Branch, Centers for Disease Control and Prevention, 1600 Clifton Road, Mailstop G14, Atlanta, GA 30333, USA.
Abstract
In March 2005, the Centers for Disease Control and Prevention (CDC) investigated a large hemorrhagic fever (HF) outbreak in Uige Province in northern Angola, West Africa. In total, 15 initial specimens were sent to CDC, Atlanta, Ga., for testing for viruses associated with viral HFs known to be present in West Africa, including ebolavirus. Marburgvirus was also included despite the fact that the origins of all earlier outbreaks were linked directly to East Africa. Surprisingly, marburgvirus was confirmed (12 of 15 specimens) as the cause of the outbreak. The outbreak likely began in October 2004 and ended in July 2005, and it included 252 cases and 227 (90%) fatalities (report from the Ministry of Health, Republic of Angola, 2005), making it the largest Marburg HF outbreak on record. A real-time quantitative reverse transcription-PCR assay utilized and adapted during the outbreak proved to be highly sensitive and sufficiently robust for field use. Partial marburgvirus RNA sequence analysis revealed up to 21% nucleotide divergence among the previously characterized East African strains, with the most distinct being Ravn from Kenya (1987). The Angolan strain was less different ( approximately 7%) from the main group of East African marburgviruses than one might expect given the large geographic separation. To more precisely analyze the virus genetic differences between outbreaks and among viruses within the Angola outbreak itself, a total of 16 complete virus genomes were determined, including those of the virus isolates Ravn (Kenya, 1987) and 05DRC, 07DRC, and 09DRC (Democratic Republic of Congo, 1998) and the reference Angolan virus isolate (Ang1379v). In addition, complete genome sequences were obtained from RNAs extracted from 10 clinical specimens reflecting various stages of the disease and locations within the Angolan outbreak. While the marburgviruses exhibit high overall genetic diversity (up to 22%), only 6.8% nucleotide difference was found between the West African Angolan viruses and the majority of East African viruses, suggesting that the virus reservoir species in these regions are not substantially distinct. Remarkably few nucleotide differences were found among the Angolan clinical specimens (0 to 0.07%), consistent with an outbreak scenario in which a single (or rare) introduction of virus from the reservoir species into the human population was followed by person-to-person transmission with little accumulation of mutations. This is in contrast to the 1998 to 2000 marburgvirus outbreak, where evidence of several virus genetic lineages (with up to 21% divergence) and multiple virus introductions into the human population was found.
========================================================================
14.) Isolation of genetically diverse Marburg viruses from Egyptian fruit bats.
========================================================================
PLoS Pathog. 2009 Jul;5(7):e1000536. doi: 10.1371/journal.ppat.1000536. Epub 2009 Jul 31.
Towner JS1, Amman BR, Sealy TK, Carroll SA, Comer JA, Kemp A, Swanepoel R, Paddock CD, Balinandi S, Khristova ML, Formenty PB, Albarino CG, Miller DM, Reed ZD, Kayiwa JT, Mills JN, Cannon DL, Greer PW, Byaruhanga E, Farnon EC, Atimnedi P, Okware S, Katongole-Mbidde E, Downing R, Tappero JW, Zaki SR, Ksiazek TG, Nichol ST, Rollin PE.
Author information
1
Special Pathogens Branch, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Abstract
In July and September 2007, miners working in Kitaka Cave, Uganda, were diagnosed with Marburg hemorrhagic fever. The likely source of infection in the cave was Egyptian fruit bats (Rousettus aegyptiacus) based on detection of Marburg virus RNA in 31/611 (5.1%) bats, virus-specific antibody in bat sera, and isolation of genetically diverse virus from bat tissues. The virus isolates were collected nine months apart, demonstrating long-term virus circulation. The bat colony was estimated to be over 100,000 animals using mark and re-capture methods, predicting the presence of over 5,000 virus-infected bats. The genetically diverse virus genome sequences from bats and miners closely matched. These data indicate common Egyptian fruit bats can represent a major natural reservoir and source of Marburg virus with potential for spillover into humans.
========================================================================
15.) Filoviruses and bats.
========================================================================
Microbiol. Aust. 2017 Mar;38(1):12-16. doi: 10.1071/MA17005. Epub 2017 Feb 17.
Schuh AJ1, Amman BR1, Towner JS1.
Author information
1
Viral Special Pathogens Branch, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, GA 30329, USA.
Abstract
While Reston and Lloviu viruses have never been associated with human disease, the other filoviruses cause outbreaks of hemorrhagic fever characterised by person-to-person transmission and high case fatality ratios. Cumulative evidence suggests that bats are the most likely reservoir hosts of the filoviruses. Ecological investigations following Marburg virus disease outbreaks associated with entry into caves inhabited by Rousettus aegyptiacus bats led to the identification of this bat species as the natural reservoir host of the marburgviruses. Experimental infection of R. aegyptiacus with Marburg virus has provided insight into the natural history of filovirus infection in bats that may help guide the search for the reservoir hosts of the ebolaviruses.
========================================================================
16.) Outbreak of Marburg hemorrhagic fever among miners in Kamwenge and Ibanda Districts, Uganda, 2007.
=======================================================================
J Infect Dis. 2011 Nov;204 Suppl 3:S796-9. doi: 10.1093/infdis/jir312.
Adjemian J1, Farnon EC, Tschioko F, Wamala JF, Byaruhanga E, Bwire GS, Kansiime E, Kagirita A, Ahimbisibwe S, Katunguka F, Jeffs B, Lutwama JJ, Downing R, Tappero JW, Formenty P, Amman B, Manning C, Towner J, Nichol ST, Rollin PE.
Author information
1
Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland 20892-2665, USA. jennifer.adjemian@nih.gov
Abstract
Marburg hemorrhagic fever was detected among 4 miners in Ibanda District, Uganda, from June through September, 2007. Infection was likely acquired through exposure to bats or bat secretions in a mine in Kamwenge District, Uganda, and possibly human-to-human transmission between some patients. We describe the epidemiologic investigation and the health education response.
========================================================================
17.) Guide to the Correct Use of Filoviral Nomenclature.
========================================================================
Curr Top Microbiol Immunol. 2017 Jun 27. doi: 10.1007/82_2017_7. [Epub ahead of print]
Kuhn JH1.
Author information
1
Integrated Research Facility at Fort Detrick (IRF-Frederick), Division of Clinical Research (DCR), National Institute of Allergy and Infectious Diseases (NIAID) National Institutes of Health (NIH), B-8200 Research Plaza, Fort Detrick, Frederick, MD, 21702, USA. kuhnjens@mail.nih.gov.
Abstract
The International Committee on Taxonomy of Viruses (ICTV) currently recognizes three genera and seven species as part of the mononegaviral family Filoviridae. Eight distinct filoviruses (Bundibugyo virus, Ebola virus, Lloviu virus, Marburg virus, Ravn virus, Reston virus, Sudan virus, and Taï Forest virus) have been assigned to these seven species. This chapter briefly summarizes the status quo of filovirus classification and focuses on the importance of differentiating between filoviral species and filoviruses and the correct use of taxonomic and vernacular filovirus names and abbreviations in written and oral discourse
========================================================================
REFERENCIAS BIBLIOGRAFICAS/ BIBLIOGRAPHICAL REFERENCES
=======================================================================
1.) Filovirus Research: How it Began.
2.) Forty-five years of Marburg virus research.
3.) Marburg haemorrhagic fever in returning travellers: an overview aimed at clinicians.
4.) Imported case of Marburg hemorrhagic fever - Colorado, 2008.
5.) Seasonal pulses of Marburg virus circulation in juvenile Rousettus aegyptiacus bats coincide with periods of increased risk of human infection.
6.) Repeated outbreaks of viral hemorrhagic fevers in Uganda.
7.) Oral shedding of Marburg virus in experimentally infected Egyptian fruit bats (Rousettus aegyptiacus).
8.) Mapping the zoonotic niche of Marburg virus disease in Africa.
9.) Is Marburg virus enzootic in Gabon?
10.) Marburg virus infection detected in a common African bat.
11.) Studies of reservoir hosts for Marburg virus.
12.) Marburgvirus Resurgence in Kitaka Mine Bat Population after Extermination Attempts, Uganda
13.) Marburgvirus genomics and association with a large hemorrhagic fever outbreak in Angola.
14.) Isolation of genetically diverse Marburg viruses from Egyptian fruit bats.
15.) Filoviruses and bats.
16.) Outbreak of Marburg hemorrhagic fever among miners in Kamwenge and Ibanda Districts, Uganda, 2007.
17.) Guide to the Correct Use of Filoviral Nomenclature.
18.) Biohazard
The Chilling True Story of the Largest Covert Biological Weapons Program in the World -- Told from Inside by the Man Who Ran It
19.) Ebola and Marburg virus vaccines.
.===============================================================
===============================================================
1.) Filovirus Research: How it Began.
===============================================================
Curr Top Microbiol Immunol. 2017 Aug 2. doi: 10.1007/82_2017_8. [Epub ahead of print]
Slenczka W1.
Author information
1
Institute of Virology, Philipps University of Marburg, Am Weinberg 19, 35037, Marburg, Germany. slenczka-marburg@t-online.de.
Abstract
The first reported filovirus outbreak occurred in August 1967, when laboratory workers in Marburg and Frankfurt, Germany, and Belgrade, Yugoslavia (now Serbia) became infected with an unknown highly pathogenic agent. The disease was characterized by high fever, malaise, rash, hemorrhagic and tetanic manifestations, and high lethality, amounting to 25%. The disease was introduced to Europe by grivets (Chlorocebus aethiops), which were used for biomedical research and vaccine production. The causative agent, Marburg virus, was isolated and identified by scientists of the University of Marburg, Germany in cooperation with specialists for viral electron microscopy at the Bernhard Nocht Institute in Hamburg, Germany. In this chapter, Dr. Slenczka, who was involved in the first isolation of Marburg virus in 1967, describes the desperate hunt of the causative agent of this first filovirus disease outbreak in the center of Europe, its successful isolation, the likely route of transmission from a monkey trading station to vaccine production facilities in Germany and Yugoslavia, and the consequences of this outbreak, including a shortage in the production of poliomyelitis vaccine In addition, this chapter provides insight into some of the peculiarities of filovirus infection, such as sexual virus transmission several months after recovery and the role of Ca2+-loss in Marburg virus pathogenesis, which were already observed during this first well-documented Marburg virus disease outbreak.
=========================================================================
2.) Forty-five years of Marburg virus research.
========================================================================
Viruses. 2012 Oct 1;4(10):1878-927. doi: 10.3390/v4101878.
Brauburger K1, Hume AJ, Mühlberger E, Olejnik J.
Author information
1
Department of Microbiology, School of Medicine and National Emerging Infectious Diseases Laboratories Institute, Boston University, Boston, MA 02118, USA. brauburk@bu.edu
Abstract
In 1967, the first reported filovirus hemorrhagic fever outbreak took place in Germany and the former Yugoslavia. The causative agent that was identified during this outbreak, Marburg virus, is one of the most deadly human pathogens. This article provides a comprehensive overview of our current knowledge about Marburg virus disease ranging from ecology to pathogenesis and molecular biology.
=========================================================================
3.) Marburg haemorrhagic fever in returning travellers: an overview aimed at clinicians.
========================================================================
Clin Microbiol Infect. 2015 Jun 22. pii: S1198-743X(15)00538-8. doi: 10.1111/1469-0691.12673. [Epub ahead of print]
Bauer MP1, Timen A2, Vossen AC3, van Dissel JT4.
Author information
1
Department of Infectious Diseases, Leiden University Medical Centre, Leiden, The Netherlands.
2
National Institute for Public Health and the Environment, Bilthoven, The Netherlands.
3
Department Medical Microbiology, Leiden University Medical Centre, Leiden, The Netherlands.
4
Department of Infectious Diseases, Leiden University Medical Centre, Leiden, The Netherlands. Electronic address: e.j.t.van_dissel@lumc.nl.
Abstract
Marburg virus haemorrhagic fever (MARV HF) is a dramatic disease that can occur in a traveller returning from an area where the virus is endemic. In this article, we provide an overview of MARV HF as an imported infection with an emphasis on clinical aspects. Although late features such as rash, signs of haemorrhagic diathesis and liver necrosis may point to the diagnosis, the initial clinical picture is non-specific. If in this early phase the patient's epidemiological exposure history is compatible with MARV HF, the patient should be isolated and managed according to viral haemorrhagic fever protocol and RT-PCR should be performed on the patient's blood as soon as possible to rule out MARV HF (or other possible viral haemorrhagic fevers). In severe cases, direct electron microscopy of blood in specialized centres (e.g. Bernhard-Nocht Institute in Hamburg, Germany) may be considered if the result of the RT-PCR is not readily available. Adequate diagnostics and empirical treatment for other acute life-threatening illnesses should not be withheld while test results are awaited, but all management and diagnostics should be weighed against the risks of nosocomial transmission.
=========================================================================
4.) Imported case of Marburg hemorrhagic fever - Colorado, 2008.
========================================================================
Centers for Disease Control and Prevention (CDC).
Abstract
Marburg hemorrhagic fever (MHF) is a rare, viral hemorrhagic fever (VHF); the causative agent is an RNA virus in the family Filoviridae, and growing evidence demonstrates that fruit bats are the natural reservoir of Marburg virus (MARV). On January 9, 2008, an infectious disease physician notified the Colorado Department of Public Health and Environment (CDPHE) of a case of unexplained febrile illness requiring hospitalization in a woman who had returned from travel in Uganda. Testing of early convalescent serum demonstrated no evidence of infection with agents that cause tropical febrile illnesses, including VHF. Six months later, in July 2008, the patient requested repeat testing after she learned of the death from MHF of a Dutch tourist who had visited the same bat-roosting cave as the patient, the Python Cave in Queen Elizabeth National Park, Uganda. The convalescent serologic testing revealed evidence of prior infection with MARV, and MARV RNA was detected in the archived early convalescent serum. A public health investigation did not identify illness consistent with secondary MHF transmission among her contacts, and no serologic evidence of infection was detected among the six tested of her eight tour companions. The patient might have acquired MARV infection through exposure to bat secretions or excretions while visiting the Python Cave. Travelers should be aware of the risk for acquiring MHF in caves or mines inhabited by bats in endemic areas in sub-Saharan Africa. Health-care providers should consider VHF among travelers returning from endemic areas who experience unexplained febrile illness.
=========================================================================
5.) Seasonal pulses of Marburg virus circulation in juvenile Rousettus aegyptiacus bats coincide with periods of increased risk of human infection.
=========================================================================
PLoS Pathog. 2012;8(10):e1002877. doi: 10.1371/journal.ppat.1002877. Epub 2012 Oct 4.
Amman BR1, Carroll SA, Reed ZD, Sealy TK, Balinandi S, Swanepoel R, Kemp A, Erickson BR, Comer JA, Campbell S, Cannon DL, Khristova ML, Atimnedi P, Paddock CD, Crockett RJ, Flietstra TD, Warfield KL, Unfer R, Katongole-Mbidde E, Downing R, Tappero JW, Zaki SR, Rollin PE, Ksiazek TG, Nichol ST, Towner JS.
Author information
1
Viral Special Pathogens Branch, Centers for Disease Control and Prevention, Atlanta, Georgia, United States of America.
Abstract
Marburg virus (family Filoviridae) causes sporadic outbreaks of severe hemorrhagic disease in sub-Saharan Africa. Bats have been implicated as likely natural reservoir hosts based most recently on an investigation of cases among miners infected in 2007 at the Kitaka mine, Uganda, which contained a large population of Marburg virus-infected Rousettus aegyptiacus fruit bats. Described here is an ecologic investigation of Python Cave, Uganda, where an American and a Dutch tourist acquired Marburg virus infection in December 2007 and July 2008. More than 40,000 R. aegyptiacus were found in the cave and were the sole bat species present. Between August 2008 and November 2009, 1,622 bats were captured and tested for Marburg virus. Q-RT-PCR analysis of bat liver/spleen tissues indicated ~2.5% of the bats were actively infected, seven of which yielded Marburg virus isolates. Moreover, Q-RT-PCR-positive lung, kidney, colon and reproductive tissues were found, consistent with potential for oral, urine, fecal or sexual transmission. The combined data for R. aegyptiacus tested from Python Cave and Kitaka mine indicate low level horizontal transmission throughout the year. However, Q-RT-PCR data show distinct pulses of virus infection in older juvenile bats (~six months of age) that temporarily coincide with the peak twice-yearly birthing seasons. Retrospective analysis of historical human infections suspected to have been the result of discrete spillover events directly from nature found 83% (54/65) events occurred during these seasonal pulses in virus circulation, perhaps demonstrating periods of increased risk of human infection. The discovery of two tags at Python Cave from bats marked at Kitaka mine, together with the close genetic linkages evident between viruses detected in geographically distant locations, are consistent with R. aegyptiacus bats existing as a large meta-population with associated virus circulation over broad geographic ranges. These findings provide a basis for developing Marburg hemorrhagic fever risk reduction strategies.
========================================================================
6.) Repeated outbreaks of viral hemorrhagic fevers in Uganda.
========================================================================
Mbonye A1, Wamala J, Winyi-Kaboyo, Tugumizemo V, Aceng J, Makumbi I.
Author information
1
Ministry of Health Head Quarters, P.O Box 7272 Kampala, Uganda. vpadmn@infocom.co.ug
Abstract
BACKGROUND:
Since the year 2000, Uganda has experienced repeated outbreaks of viral hemorrhagic fevers (VHF). Ebola VHF outbreak occurred in the districts of Gulu in 2000, Bundibugyo, 2007, Luwero, 2011, Kibaale in July 2012, Luwero in November 2012. Marburg VHF was earlier reported in Ibanda in 2007. More recently in 2012, two outbreaks of Marburg VHF have occurred in Ibanda and Kabale districts.
OBJECTIVE:
To present the epidemiological picture of the Marburg VHF recently reported in Ibanda and Kabale districts and propose research questions to generate evidence to mitigate future epidemics.
METHODS:
A case definition for a VHF was developed. A frequency distribution of symptoms of confirmed and probable cases was done. Descriptive analyses of reported cases using simple percentages, percent distributions and computation of means was performed.
RESULTS:
The Marburg epidemic was reported in early September and by November 2012, a cumulative of 14 cases (9 confirmed and 5 probable) including 7 deaths had been registered, giving a case fatality rate (CFR) of 50%. A total of 202 contacts had been listed; out of which 193 had completed the 21-day follow-up period. The index case was a 33-year old male, a teacher at Nyakatukura Secondary School in Ibanda district. He travelled to Ibanda from Kabale, his home district on 31st August 2012, reportedly healthy. He fell sick on 3rd September 2012 with complaints of fever, headache, loss of appetite and general body weakness. Overall, the dominant symptoms for all cases were fever, vomiting, loss of appetite, headache, abdominal pain, fatigue, diarrhea, and the least in occurrence was bleeding which accounted for 35.5% of all the cases.
CONCLUSION:
The source of infection for all the five Ebola Hemorrhagic fever outbreaks in Uganda and the recent Marburg VHF outbreak in Ibanda and Kabale is not known. Currently there is suspicion that there could be an animal reservoir of the Ebola and Marburg viruses from where occasional spillage into the human population occurs resulting in disease outbreaks. This and other hypotheses require further investigation.
========================================================================
7.) Oral shedding of Marburg virus in experimentally infected Egyptian fruit bats (Rousettus aegyptiacus).
========================================================================
J Wildl Dis. 2015 Jan;51(1):113-24. doi: 10.7589/2014-08-198.
Amman BR1, Jones ME, Sealy TK, Uebelhoer LS, Schuh AJ, Bird BH, Coleman-McCray JD, Martin BE, Nichol ST, Towner JS.
Author information
1
1 Centers for Disease Control and Prevention, Viral Special Pathogens Branch, 1600 Clifton Rd. NE, Atlanta, Georgia 30333, USA.
Abstract
Marburg virus (Marburg marburgvirus; MARV) causes sporadic outbreaks of Marburg hemorrhagic fever (MHF) in Africa. The Egyptian fruit bat (Rousettus aegyptiacus) has been identified as a natural reservoir based most-recently on the repeated isolation of MARV directly from bats caught at two locations in southwestern Uganda where miners and tourists separately contracted MHF from 2007-08. Despite learning much about the ecology of MARV through extensive field investigations, there remained unanswered questions such as determining the primary routes of virus shedding and the severity of disease, if any, caused by MARV in infected bats. To answer these questions and others, we experimentally infected captive-bred R. aegyptiacus with MARV under high (biosafety level 4) containment. These experiments have shown infection profiles consistent with R. aegyptiacus being a bona fide natural reservoir host for MARV and demonstrated routes of viral shedding capable of infecting humans and other animals.
========================================================================
8.) Mapping the zoonotic niche of Marburg virus disease in Africa.
=======================================================================
Trans R Soc Trop Med Hyg. 2015 Jun;109(6):366-78. doi: 10.1093/trstmh/trv024. Epub 2015 Mar 27.
Pigott DM1, Golding N2, Mylne A2, Huang Z2, Weiss DJ2, Brady OJ2, Kraemer MU2, Hay SI3.
Author information
1
Spatial Ecology & Epidemiology Group, Department of Zoology, University of Oxford, Oxford, UK david.pigott@zoo.ox.ac.uk.
2
Spatial Ecology & Epidemiology Group, Department of Zoology, University of Oxford, Oxford, UK.
3
Spatial Ecology & Epidemiology Group, Department of Zoology, University of Oxford, Oxford, UK Fogarty International Center, National Institutes of Health, Bethesda, Maryland, USA.
Abstract
BACKGROUND:
Marburg virus disease (MVD) describes a viral haemorrhagic fever responsible for a number of outbreaks across eastern and southern Africa. It is a zoonotic disease, with the Egyptian rousette (Rousettus aegyptiacus) identified as a reservoir host. Infection is suspected to result from contact between this reservoir and human populations, with occasional secondary human-to-human transmission.
METHODS:
Index cases of previous human outbreaks were identified and reports of infection in animals recorded. These data were modelled within a species distribution modelling framework in order to generate a probabilistic surface of zoonotic transmission potential of MVD across sub-Saharan Africa.
RESULTS:
Areas suitable for zoonotic transmission of MVD are predicted in 27 countries inhabited by 105 million people. Regions are suggested for exploratory surveys to better characterise the geographical distribution of the disease, as well as for directing efforts to communicate the risk of practices enhancing zoonotic contact.
CONCLUSIONS:
These maps can inform future contingency and preparedness strategies for MVD control, especially where secondary transmission is a risk. Coupling this risk map with patient travel histories could be used to guide the differential diagnosis of highly transmissible pathogens, enabling more rapid response to outbreaks of haemorrhagic fever.
========================================================================
9.) Is Marburg virus enzootic in Gabon?
========================================================================
J Infect Dis. 2011 Nov;204 Suppl 3:S800-3. doi: 10.1093/infdis/jir358.
Maganga GD1, Bourgarel M, Ella GE, Drexler JF, Gonzalez JP, Drosten C, Leroy EM.
Author information
1
Centre International de Recherches Médicales de Franceville, Gabon.
Abstract
Marburg virus (MARV) nucleic acid was detected in Rousettus aegyptiacus bats in 2005 and 2006 in the midwest and southeast of Gabon. In this study we used MARV-specific real-time reverse-transcription polymerase chain reaction (RT-PCR) and MARV-specific nested RT-PCR assay to screen 1257 bats caught during July 2009, December 2009, and June 2010 in 3 caves situated in northern Gabon. Nine specimens tested positive by the real-time assay, with cycle threshold values ranging from 35 to 39, of which only 1 R. aegyptiacus specimen collected in 2009 was positive in the nested VP35 RT-PCR assay. Together with MARV-positive bats in the south and west found in 2005 and 2006, confirmation of phylogenetically closely related MARV-positive bats 5 years later and in northern Gabon suggests that MARV is now enzootic in Gabon and emphasizes the importance of long-term monitoring of bat populations and human-bat interfaces.
========================================================================
10.) Marburg virus infection detected in a common African bat.
========================================================================
PLoS One. 2007 Aug 22;2(8):e764.
Towner JS1, Pourrut X, Albariño CG, Nkogue CN, Bird BH, Grard G, Ksiazek TG, Gonzalez JP, Nichol ST, Leroy EM.
Author information
1
Centers for Disease Control and Prevention, Special Pathogens Branch, Atlanta, Georgia, United States of America.
Abstract
Marburg and Ebola viruses can cause large hemorrhagic fever (HF) outbreaks with high case fatality (80-90%) in human and great apes. Identification of the natural reservoir of these viruses is one of the most important topics in this field and a fundamental key to understanding their natural history. Despite the discovery of this virus family almost 40 years ago, the search for the natural reservoir of these lethal pathogens remains an enigma despite numerous ecological studies. Here, we report the discovery of Marburg virus in a common species of fruit bat (Rousettus aegyptiacus) in Gabon as shown by finding virus-specific RNA and IgG antibody in individual bats. These Marburg virus positive bats represent the first naturally infected non-primate animals identified. Furthermore, this is the first report of Marburg virus being present in this area of Africa, thus extending the known range of the virus. These data imply that more areas are at risk for MHF outbreaks than previously realized and correspond well with a recently published report in which three species of fruit bats were demonstrated to be likely reservoirs for Ebola virus.
========================================================================
11.) Studies of reservoir hosts for Marburg virus.
========================================================================
Emerg Infect Dis. 2007 Dec;13(12):1847-51. doi: 10.3201/eid1312.071115.
Swanepoel R1, Smit SB, Rollin PE, Formenty P, Leman PA, Kemp A, Burt FJ, Grobbelaar AA, Croft J, Bausch DG, Zeller H, Leirs H, Braack LE, Libande ML, Zaki S, Nichol ST, Ksiazek TG, Paweska JT; International Scientific and Technical Committee for Marburg Hemorrhagic Fever Control in the Democratic Republic of Congo.
Author information
1
National Institute for Communicable Diseases, Sandringham, Republic of South Africa. bobs@nicd.ac.za
Abstract
To determine reservoir hosts for Marburg virus (MARV), we examined the fauna of a mine in northeastern Democratic Republic of the Congo. The mine was associated with a protracted outbreak of Marburg hemorrhagic fever during 1998-2000. We found MARV nucleic acid in 12 bats, comprising 3.0%-3.6% of 2 species of insectivorous bat and 1 species of fruit bat. We found antibody to the virus in the serum of 9.7% of 1 of the insectivorous species and in 20.5% of the fruit bat species, but attempts to isolate virus were unsuccessful
========================================================================
12.) Marburgvirus Resurgence in Kitaka Mine Bat Population after Extermination Attempts, Uganda
=======================================================================
Emerg Infect Dis. 2014 Oct; 20(10): 1761–1764. doi: 10.3201/eid2010.140696
Brian R. Amman, Luke Nyakarahuka, Anita K. McElroy, Kimberly A. Dodd, Tara K. Sealy, Amy J. Schuh, Trevor R. Shoemaker, Stephen Balinandi, Patrick Atimnedi, Winyi Kaboyo, Stuart T. Nichol, and Jonathan S. Townercorresponding author
To the Editor: Marburg virus (MARV) and Ravn virus (RAVV), collectively called marburgviruses, cause Marburg hemorrhagic fever (MHF) in humans. In July 2007, 4 cases of MHF (1 fatal) occurred in miners at Kitaka Mine in southern Uganda. Later, MHF occurred in 2 tourists who visited Python Cave, ≈50 km from Kitaka Mine. One of the tourists was from the United States (December 2007) and 1 was from the Netherlands (July 2008); 1 case was fatal (1,2,3). The cave and the mine each contained 40,000–100,000 Rousettus aegyptiacus bats (Egyptian fruit bats).
Longitudinal investigations of the outbreaks at both locations were initiated by the Viral Special Pathogens Branch of the Centers for Disease Control and Prevention (CDC, Atlanta, GA, USA, and Entebbe, Uganda) in collaboration with the Uganda Wildlife Authority (UWA) and the Uganda Virus Research Institute (UVRI). During these studies, genetically diverse MARVs and RAVVs were isolated directly from bat tissues, and infection levels of the 2 viruses were found to increase in juvenile bats on a predictable bi-annual basis (4,5). However, investigations at Kitaka Mine were stopped when the miners exterminated the bat colony by restricting egress from the cave with papyrus reed barriers and then entangling the bats in fishing nets draped over the exits. The trapping continued for weeks, and the entrances were then sealed with sticks and plastic. These depopulation efforts were documented by researchers from UVRI, the CDC, the National Institute of Communicable Diseases (Sandringham, South Africa), and UWA during site visits to Kitaka Mine (Technical Appendix Figure). In August 2008, thousands of dead bats were found piled in the forest, and by November 2008, there was no evidence of bats living in the mine; whether 100% extermination was achieved is unknown. CDC, UVRI, and UWA recommended against extermination, believing that any results would be temporary and that such efforts could exacerbate the problem if bat exclusion methods were not complete and permanent (6,7).
In October 2012, the most recent known marburgvirus outbreak was detected in Ibanda, a town in southwest Uganda. Ibanda is ≈20 km from the Kitaka Mine and is the urban center that serves smaller communities in the Kitaka area. This MHF outbreak was the largest in Ugandan history: 15 laboratory-confirmed cases occurred (8). In November 2012, an ecologic investigation of the greater Ibanda/Kitaka area was initiated. The investigation included interviews with local authorities to locate all known R. aegyptiacus colonies in the area. Although minor colonies of small insectivorous bats were found, the only identifiable colony of R. aegyptiacus bats was found inside the re-opened Kitaka Mine, albeit at much reduced size, perhaps 1%–5% of that found before depopulation efforts.
To determine whether the R. aegyptiacus bats that had repopulated Kitaka Mine were actively infected with marburgviruses, we tested 400 bats by using previously described methods (4,5). Viral RNA was extracted from ≈100 mg of liver and spleen tissue by using the MagMAX Total Nucleic Acid Isolation Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s recommended protocol. The Fisher exact test was conducted by using IBM SPSS Statistics, version 19.0 (IBM Corp., Armonk, NY, USA).
Of the 400 R. aegyptiacus bats collected, 53 (13.3%) were positive for marburgvirus RNA by quantitative reverse transcription PCR (32/233 [13.7%] adults and 21/167 [12.6%] juveniles; Technical Appendix Table); marburgvirus was isolated from tissue samples from 9 of the 400 bats. The overall level of active infection was significantly higher than that found in Kitaka Mine during 2007–2008 (5.1%) (5) (Fisher exact test, p<0.001) and in other studies in Uganda (Python Cave [2.5%]) and Gabon (4.8%) (4,9). The reason for the increase is not clear, but it may be related to the effects of the extermination and subsequent repopulation. Increases in disease prevalence in wildlife populations after culling are not unprecedented (6,7). We speculate that after the depopulation attempt, a pool of susceptible bats became established over time and was subjected to multiple marburgvirus introductions, as evidenced by the genetic diversity of viruses isolated from the bats (Figure). A pool of susceptible bats would have led to higher levels of active infection within the colony, thereby increasing the potential for virus spillover into the human population. A significant sex and age bias was not detected with respect to active infection during the breeding season (Fisher exact test, p>0.5 for both), and overall, the presence of virus-specific IgG among the bats was 16.5%, a finding consistent with that in previous studies (4,5).
Phylogeny of concatenated marburgvirus nucleoprotein (NP) and viral protein 35 (VP35) gene fragments as determined by using the maximum-likelihood method. Sequences from the NP (289–372 nt) and VP35 (203–213 nt) genes were amplified and ...
Phylogenetic analysis of viral RNA genome fragment sequences in this study showed high marburgvirus genetic diversity, including the presence of RAVVs and MARVs. Sequences for isolates from 3 bats were nearly identical to those of the MARV isolates obtained from patients in the 2012 Ibanda outbreak (8), suggesting that bats from Kitaka Mine were a likely source of the virus.
========================================================================
13.) Marburgvirus genomics and association with a large hemorrhagic fever outbreak in Angola.
=======================================================================
J Virol. 2006 Jul;80(13):6497-516.
Towner JS1, Khristova ML, Sealy TK, Vincent MJ, Erickson BR, Bawiec DA, Hartman AL, Comer JA, Zaki SR, Ströher U, Gomes da Silva F, del Castillo F, Rollin PE, Ksiazek TG, Nichol ST.
Author information
1
Special Pathogens Branch, Centers for Disease Control and Prevention, 1600 Clifton Road, Mailstop G14, Atlanta, GA 30333, USA.
Abstract
In March 2005, the Centers for Disease Control and Prevention (CDC) investigated a large hemorrhagic fever (HF) outbreak in Uige Province in northern Angola, West Africa. In total, 15 initial specimens were sent to CDC, Atlanta, Ga., for testing for viruses associated with viral HFs known to be present in West Africa, including ebolavirus. Marburgvirus was also included despite the fact that the origins of all earlier outbreaks were linked directly to East Africa. Surprisingly, marburgvirus was confirmed (12 of 15 specimens) as the cause of the outbreak. The outbreak likely began in October 2004 and ended in July 2005, and it included 252 cases and 227 (90%) fatalities (report from the Ministry of Health, Republic of Angola, 2005), making it the largest Marburg HF outbreak on record. A real-time quantitative reverse transcription-PCR assay utilized and adapted during the outbreak proved to be highly sensitive and sufficiently robust for field use. Partial marburgvirus RNA sequence analysis revealed up to 21% nucleotide divergence among the previously characterized East African strains, with the most distinct being Ravn from Kenya (1987). The Angolan strain was less different ( approximately 7%) from the main group of East African marburgviruses than one might expect given the large geographic separation. To more precisely analyze the virus genetic differences between outbreaks and among viruses within the Angola outbreak itself, a total of 16 complete virus genomes were determined, including those of the virus isolates Ravn (Kenya, 1987) and 05DRC, 07DRC, and 09DRC (Democratic Republic of Congo, 1998) and the reference Angolan virus isolate (Ang1379v). In addition, complete genome sequences were obtained from RNAs extracted from 10 clinical specimens reflecting various stages of the disease and locations within the Angolan outbreak. While the marburgviruses exhibit high overall genetic diversity (up to 22%), only 6.8% nucleotide difference was found between the West African Angolan viruses and the majority of East African viruses, suggesting that the virus reservoir species in these regions are not substantially distinct. Remarkably few nucleotide differences were found among the Angolan clinical specimens (0 to 0.07%), consistent with an outbreak scenario in which a single (or rare) introduction of virus from the reservoir species into the human population was followed by person-to-person transmission with little accumulation of mutations. This is in contrast to the 1998 to 2000 marburgvirus outbreak, where evidence of several virus genetic lineages (with up to 21% divergence) and multiple virus introductions into the human population was found.
========================================================================
14.) Isolation of genetically diverse Marburg viruses from Egyptian fruit bats.
========================================================================
PLoS Pathog. 2009 Jul;5(7):e1000536. doi: 10.1371/journal.ppat.1000536. Epub 2009 Jul 31.
Towner JS1, Amman BR, Sealy TK, Carroll SA, Comer JA, Kemp A, Swanepoel R, Paddock CD, Balinandi S, Khristova ML, Formenty PB, Albarino CG, Miller DM, Reed ZD, Kayiwa JT, Mills JN, Cannon DL, Greer PW, Byaruhanga E, Farnon EC, Atimnedi P, Okware S, Katongole-Mbidde E, Downing R, Tappero JW, Zaki SR, Ksiazek TG, Nichol ST, Rollin PE.
Author information
1
Special Pathogens Branch, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Abstract
In July and September 2007, miners working in Kitaka Cave, Uganda, were diagnosed with Marburg hemorrhagic fever. The likely source of infection in the cave was Egyptian fruit bats (Rousettus aegyptiacus) based on detection of Marburg virus RNA in 31/611 (5.1%) bats, virus-specific antibody in bat sera, and isolation of genetically diverse virus from bat tissues. The virus isolates were collected nine months apart, demonstrating long-term virus circulation. The bat colony was estimated to be over 100,000 animals using mark and re-capture methods, predicting the presence of over 5,000 virus-infected bats. The genetically diverse virus genome sequences from bats and miners closely matched. These data indicate common Egyptian fruit bats can represent a major natural reservoir and source of Marburg virus with potential for spillover into humans.
========================================================================
15.) Filoviruses and bats.
========================================================================
Microbiol. Aust. 2017 Mar;38(1):12-16. doi: 10.1071/MA17005. Epub 2017 Feb 17.
Schuh AJ1, Amman BR1, Towner JS1.
Author information
1
Viral Special Pathogens Branch, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, GA 30329, USA.
Abstract
While Reston and Lloviu viruses have never been associated with human disease, the other filoviruses cause outbreaks of hemorrhagic fever characterised by person-to-person transmission and high case fatality ratios. Cumulative evidence suggests that bats are the most likely reservoir hosts of the filoviruses. Ecological investigations following Marburg virus disease outbreaks associated with entry into caves inhabited by Rousettus aegyptiacus bats led to the identification of this bat species as the natural reservoir host of the marburgviruses. Experimental infection of R. aegyptiacus with Marburg virus has provided insight into the natural history of filovirus infection in bats that may help guide the search for the reservoir hosts of the ebolaviruses.
========================================================================
16.) Outbreak of Marburg hemorrhagic fever among miners in Kamwenge and Ibanda Districts, Uganda, 2007.
=======================================================================
J Infect Dis. 2011 Nov;204 Suppl 3:S796-9. doi: 10.1093/infdis/jir312.
Adjemian J1, Farnon EC, Tschioko F, Wamala JF, Byaruhanga E, Bwire GS, Kansiime E, Kagirita A, Ahimbisibwe S, Katunguka F, Jeffs B, Lutwama JJ, Downing R, Tappero JW, Formenty P, Amman B, Manning C, Towner J, Nichol ST, Rollin PE.
Author information
1
Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland 20892-2665, USA. jennifer.adjemian@nih.gov
Abstract
Marburg hemorrhagic fever was detected among 4 miners in Ibanda District, Uganda, from June through September, 2007. Infection was likely acquired through exposure to bats or bat secretions in a mine in Kamwenge District, Uganda, and possibly human-to-human transmission between some patients. We describe the epidemiologic investigation and the health education response.
========================================================================
17.) Guide to the Correct Use of Filoviral Nomenclature.
========================================================================
Curr Top Microbiol Immunol. 2017 Jun 27. doi: 10.1007/82_2017_7. [Epub ahead of print]
Kuhn JH1.
Author information
1
Integrated Research Facility at Fort Detrick (IRF-Frederick), Division of Clinical Research (DCR), National Institute of Allergy and Infectious Diseases (NIAID) National Institutes of Health (NIH), B-8200 Research Plaza, Fort Detrick, Frederick, MD, 21702, USA. kuhnjens@mail.nih.gov.
Abstract
The International Committee on Taxonomy of Viruses (ICTV) currently recognizes three genera and seven species as part of the mononegaviral family Filoviridae. Eight distinct filoviruses (Bundibugyo virus, Ebola virus, Lloviu virus, Marburg virus, Ravn virus, Reston virus, Sudan virus, and Taï Forest virus) have been assigned to these seven species. This chapter briefly summarizes the status quo of filovirus classification and focuses on the importance of differentiating between filoviral species and filoviruses and the correct use of taxonomic and vernacular filovirus names and abbreviations in written and oral discourse
18.) Biohazard
The Chilling True Story of the Largest Covert Biological Weapons Program in the World -- Told from Inside by the Man Who Ran It
========================================================================
By KEN ALIBEK with STEPHEN HANDELMAN
Source:http://www.nytimes.com/books/99/06/20/reviews/990620.20taubmat.html
Tularemia is a highly infectious disease that produces headaches, nausea and high fevers. It can be lethal if untreated. Tularemia is also hard to extinguish, making it attractive to anyone trying to produce biological weapons.
That's just what Ken Alibek was doing for the Soviet Union in 1983 when he found himself standing in a puddle of tularemia bacteria that had accidentally spilled onto the floor of a secret weapons lab. There was enough tularemia in the small, milky brown pool to infect everyone in the Soviet Union. Within hours Alibek was too sick to move. Only megadoses of tetracycline, hastily obtained from a friend, prevented the disease from disabling if not killing him.
That is one of many harrowing moments that Alibek describes in this absorbing account of the Soviet Union's demonic effort to make biological weapons. The program was one of the best-kept Russian secrets of the cold war, and Alibek was one of its central architects. He reports that at its high point in the late 1980's, when Mikhail Gorbachev was the Soviet leader, the program consumed close to $1 billion a year and employed more than 60,000 people at dozens of clandestine sites. Needless to say, it was not an activity that Gorbachev advertised as he tried to improve relations with the West.
Though the Russian effort is now believed to be largely abandoned, biological weapons remain a threat, perhaps even a greater one today because they can be made relatively easily and inexpensively by terrorist groups and leaders like Saddam Hussein.
Alibek, born Kanatjan Alibekov, defected to the United States in 1992 and changed his name. By then he had quit the weapons project in disgust, but for nearly all his career as a Soviet scientist he excelled at the grim business of cultivating biological agents and adapting them for use in missiles and bombs. For many years he was deputy director of Biopreparat, an ostensibly civilian agency that was actually involved in advanced research into biological weapons. Alibek provided American officials with their first full description of the Soviet effort when he defected.
In ''Biohazard'' he performs the same service for readers, with a strong writing assist from Stephen Handelman, who was a Moscow correspondent for The Toronto Star. The book works best as a richly descriptive report on the Soviet program and Alibek's role in it. It is less successful as a portrait of Alibek and his transition from germ warfare acolyte to apostate.
The story is sobering. With no limit to the resources it was prepared to invest in unconventional weapons research, the Soviet Union developed an extensive arsenal of deadly pathogens, including anthrax, smallpox, plague, brucellosis and tularemia. Tons of these bacteria and viruses were churned out at production centers, often in vaccine-resistant strains that could be effectively dispersed in liquid, powder or aerosol form. Moscow even tried to manipulate the AIDS virus so it could be used as a weapon. The disease's long incubation period made it unsuitable.
For Alibek and his colleagues, the grotesque work was just another day at the office. He recalls a meeting in 1988 at Soviet Army headquarters in Moscow, where he was instructed to arm long-range missiles with deadly germs. ''I made a few quick calculations on my note pad,'' he says. ''At least 400 kilograms of anthrax, prepared in dry form for use as an aerosol, would be required for 10 warheads.'' Martha Stewart couldn't have put it more innocuously.
The Kremlin went ahead with such work even though it had signed the 1972 Biological Weapons Convention, which banned the development, production and stockpiling of biological agents for offensive military purposes. Just a year after signing the accord, the Soviet Government secretly initiated an effort to modernize its biological weapons and to invent new ones. The United States, for its part, maintained a robust biological warfare program until 1969, when President Richard Nixon renounced the use of such weapons and restricted research to defensive measures like immunization.
Alibek was drawn into the Soviet campaign in 1975, deflected from a conventional career as a military physician by the allure of highly classified research, the prospect of rapid advancement and the mistaken belief that the Soviet Union had no choice but to keep pace with the United States in germ warfare technology. He was a Kazakh native eager to prove himself to his Russian superiors. With a knack for epidemiology and laboratory research, he was soon building what he describes matter-of-factly as ''the world's most efficient assembly line for the mass production of weaponized anthrax.''
Alibek has a fine eye for the cold-blooded customs of the Soviet state, including coercion and deception. He never told his supervisors about his frightening bout with tularemia, fearing it would cost his job. A few years earlier, while a student at the Tomsk Medical Institute in Siberia, he had surmised from medical records that Soviet forces had used the same disease as a weapon against German troops outside Stalingrad in 1942. His professor, a colonel, icily told Alibek, ''You have gone beyond your assignment,'' and advised him never to speak of the matter again.
Though Alibek struggles to explain his enthusiasm for biological weapons work, he seems reluctant to probe beyond surface emotions. He stops the narrative periodically for moments of introspection like this: ''I still shuddered occasionally when I looked at the bacteria multiplying in our fermenters and considered that they could end the lives of millions of people. But the secret culture of our labs had changed my outlook. My parents would not have recognized the man I had become.'' Unhappily, these tantalizing passages are but brief digressions, leaving one to puzzle over just why Alibek turned against the system.
The Russian program was theoretically dismantled in recent years at the order of President Boris N. Yeltsin, but Ken Alibek makes clear there may still be active remnants. Given the unblinking support he and thousands of others gave to the effort, that would not be surprising.
===========================================================================
19.) Ebola and Marburg virus vaccines.
===========================================================================
Virus Genes. 2017 Aug;53(4):501-515. doi: 10.1007/s11262-017-1455-x. Epub 2017 Apr 26.
Reynolds P1, Marzi A2.
Author information
1
Laboratory of Virology, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Hamilton, MT, USA.
2
Laboratory of Virology, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Hamilton, MT, USA. marzia@niaid.nih.gov.
Abstract
The filoviruses, Ebola virus (EBOV), and Marburg virus (MARV), are among the most pathogenic viruses known to man and the causative agents of viral hemorrhagic fever outbreaks in Africa with case fatality rates of up to 90%. Nearly 30,000 infections were observed in the latest EBOV epidemic in West Africa; previous outbreaks were much smaller, typically only affecting less than a few hundred people. Compared to other diseases such as AIDS or Malaria with millions of cases annually, filovirus hemorrhagic fever (FHF) is one of the neglected infectious diseases. There are no licensed vaccines or therapeutics available to treat EBOV and MARV infections; therefore, these pathogens can only be handled in maximum containment laboratories and are classified as select agents. Under these limitations, a very few laboratories worldwide conducted basic research and countermeasure development for EBOV and MARV since their respective discoveries in 1967 (MARV) and 1976 (EBOV). In this review, we discuss several vaccine platforms against EBOV and MARV, which have been assessed for their protective efficacy in animal models of FHF. The focus is on the most promising approaches, which were accelerated in clinical development (phase I-III trials) during the EBOV epidemic in West Africa.
Producido Por Dr. José Lapenta R. Dermatólogo
Maracay Estado Aragua Venezuela 2.017-2.023
Telf: 04142976087- 04127766810
04166401045
Follow @dermagicexpress



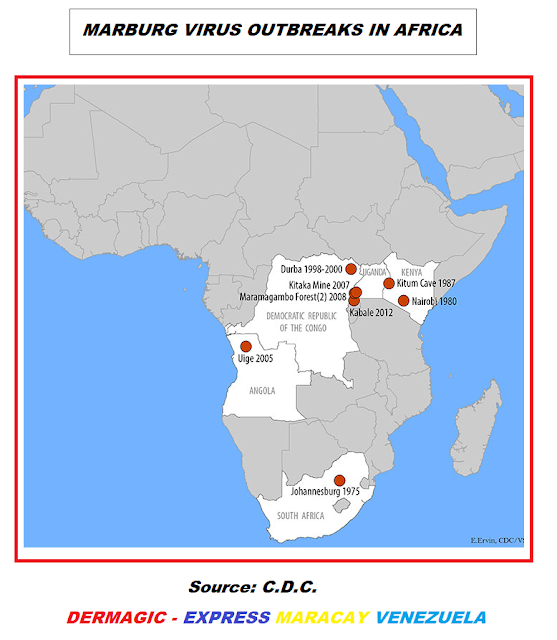


No hay comentarios:
Publicar un comentario
Tu comentario será objeto de revisión y luego aprobado.
Your comment will be revised and then approved.