La Pitiriasis Versicolor, Mitos, Historia y Tratamientos. !
The Pityriasis Versicolor, Myths, History, and Treatments. !
PITIRIASIS VERSICOLOR
=================
Hola amigos de la red, DERMAGIC EXPRESS hoy les trae un tema bien interesante, porque es muy común en la práctica médica dermatológica: LA PITIRIASIS VERSICOLOR, REVISION también conocida como TIÑA VERSICOLOR, enfermedad que en la antigüedad fue conocida bajo el nombre de "MANCHAS DEL HIGADO", por la creencia de que el daño hepático era el causal de ella.
Esta es la razón principal de esta Revisión Bibliográfica: la mayoría de los pacientes que acuden al médico piensan que esta enfermedad es producida por un "DAÑO HEPATICO" o abuso de alcohol, y el nombre de "MANCHAS DEL HIGADO" se lo colocaron porque el consumo de bebidas alcohólicas, por el efecto de vasodilatación en la piel, hace que las manchas se "noten" mas, eso es todo.
También son conocidas como "HONGO SOLAR" porque cuando la persona contaminada expone su piel al sol (PISCINA O PLAYA) se notan mas las lesiones, y realmente las manchas ya están allí, pero producen un efecto "PARAGUA" y la piel no se broncea en el área del hongo.
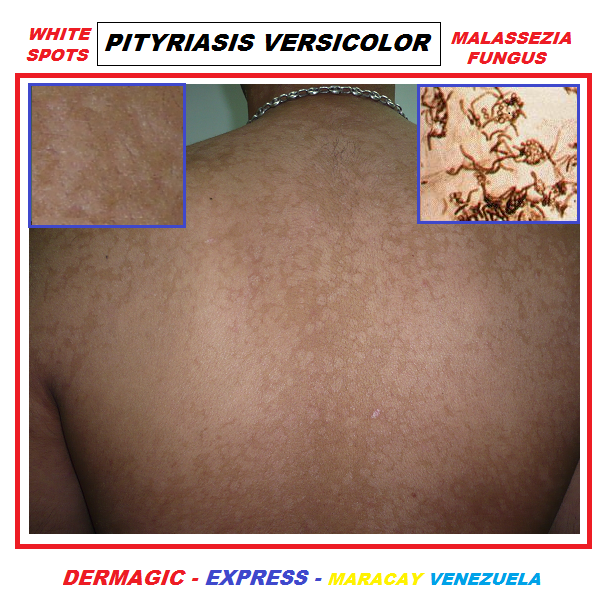
PITIRIASIS VERSICOLOR SENOS MUJER
La enfermedad se manifiesta como unas "manchas" ovales de pequeño a mediano tamaño, las cuales pueden ser de varios colores: BLANCAS, ROSADAS Y MARRONES, de allí el nombre de VERSICOLOR, que significa "DE VARIOS COLORES", por lo general se presentan desde el área del cuero cabelludo, bajando por el cuello hombro, tórax y brazos. En algunos casos producen picazón o prurito, pero mayormente son asintomáticas.
DIAGNOSTICO DE LA PITIRIASIS VERSICOLOR:
=========================================
1.) CLÍNICAMENTE: el aspecto de las lesiones.
2.) SIGNO DE BESNIER: al rasparlas con la uña producen descamación.
3.) CINTA ADHESIVA: Consiste en colocar un teipe transparente "SCOTCH" sobre la lesión, retirarlo, pegarlo a una laminilla y observar al microscopio óptico las formas del hongo descritas como "espaguetis con bolas de carne o albóndigas"
4.) KOH: HIDROXIDO DE POTASIO AL 10%: o tinta AZUL PARKER, la cual se coloca en los bordes del teipe adherido a la lámina, esto permite mejor la visualización del hongo.
5.) LUZ DE WOOD: viejo método que data de 1.925 cuando los científicos MARGAROT Y DEVEZE descubrieron que la luz ultravioleta con un filtro de WOOD permitía ver "FLUORESCENCIA" en algunas enfermedades, en este caso: al colocar el paciente en un cuarto oscuro y alumbrarlo con la lámpara de Wood: las lesiones toman un color "VIOLETA". Esta lámpara es usada comúnmente hoy día para verificar "BILLETES FALSOS".
6.) CULTIVO: en la práctica diaria no se utiliza, su utilidad es desde el punto de vista científico para la tipificación del hongo y sus variantes, hoy día se describen más de 13 variantes de MALASSEZIA, siendo la FURFUR Y GLOBOSA, las más involucradas en el humano.
DIAGNOSTICO DIFERENCIAL:
=========================
LA PITIRIASIS VERSICOLOR SE PUEDE CONFUNDIR CON:
1.) LEPRA INDETERMINADA.
2.) PITIRIASIS ALBA.
3.) VITILIGO.
4.) PITIRIASIS ROSADA.
5.) DERMATITIS SEBORREICA.
6.) HIPOMELANOSIS MACULAR PROGRESIVA.
7.) ERITRASMA.
8.) SÍFILIS.
No todas las personas expuestas al hongo se contaminan, y esto sucede porque la COMPOSICIÓN QUÍMICA DE LA PIEL, VARIA DE PERSONA A PERSONA, quiere decir esto que hay personas que NUNCA SUFRIRÁN DE PITIRIASIS VERSICOLOR, aun teniendo familiares y personas cercanas con la enfermedad, pues su COMPOSICIÓN LIPÍDICA, de la piel no es afín con el hongo.
PITIRIASIS VERSICOLOR GIGANTE EN ESPALDA DE MUJER
LOS TRATAMIENTOS MAS UTILIZADOS:
==================================
1.) HIPOSULFITO DE SODIO.
2.) TOLNAFTATO.
3.) KETOCONAZOL TABLETAS Y CHAMPU.
4.) TERBINAFINA.
5.) FLUCONAZOL.
6.) ITRACONAZOL.
7.) SERTACONAZOL.
8.) ACIDO SALICILICO
9.) FENOL.
10.) BIFONAZOL.
11.) SULFURO DE SELENIO CHAMPU y otros
12.) CICLOPIROXOLAMINA.
De estos los más populares son KETOCONAZOL en tabletas y champú, ITRACONAZOLE, TERBINAFINA Y FOLUCONAZOL oral, Y SULFURO DE SELENIO champú.
Debes entender que una vez realizado el tratamiento debes exponer tu piel al sol: PISCINA O PLAYA, paradójicamente, PUES si la piel ya sana no recibe luz solar, tardara mas en tomar su coloración original. El hongo inhibe la coloración normal de la piel produciendo HIPOCROMIA RESIDUAL.
Esta enfermedad, siendo totalmente benigna suele RECAER CON FRECUENCIA y esto se debe al hecho que siempre tu piel estará expuesta al hongo en PISCINAS, PLAYAS, PERSONAS o ROPA contaminada, y ella por su composición química como te explique, en las personas afectadas tiene afinidad por el hongo.
Como mantenimiento luego de curar de la enfermedad te recomiendo utilizar un buen champú anti caspa que contenga un antimicótico como KETOCONAZOL, BIFONAZOL o SULFURO DE SELENIO, una o 2 veces semanal. Esto por el hecho que ya te explique, el hongo tiene su "HABITAT" en el cuero cabelludo.
En las referencias los hechos, en las fotos distintas formas DE LA PITIRIASIS VERSICOLOR.
Saludos a Todos.
Dr. José Lapenta.
Dr. José M. Lapenta
===================
Hello friends of the network, DERMAGIC EXPRESS today brings you a very interesting topic, because it is very common in the dermatological medical practice: PIYIRIASIS VERSICOLOR, A REVIEW, also known as TINEA VERSICOLOR, a disease that in ancient times was known as "LIVER SPOTS "because of the belief that liver damage was the cause of it.
This is the main reason for this Bibliographic Review: Most patients who come to the doctor think that this disease is caused by a "HEPATIC DAMAGE" or alcohol abuse, and the name "LIVER SPOTS" was placed because the Consumption of alcoholic beverages, by the effect of vasodilatation on the skin, makes the spots "notice" more, that's all.
They are also known as "SUN FUNGUS" because when the contaminated person exposes their skin to the sun (SWIMMING POOL or BEACH) the lesions are noticed, and indeed the spots are already there, but they produce a "UMBRELLA" effect and the skin does not tan In the mushroom area.
In 1.889 the scientist ROBIN (Baillon) described the causal agent of this pathology THE MALASSEZIA FURFUR, also known today as PITYROSPORUM ORBICULARE, and its other variant PITYROSPORUM OVALE, a superficial fungus with a great affinity for "skin fats”, which means that it is LIPOPHILIC. This fungus once infected the person is settled or lives on the scalp. Recall that in recent years this fungus has been linked with SEBORRHEIC DERMATITIS or "DANDRUFF".

The disease manifests itself as an oval "spot" of small to medium size, which can be of several colors: WHITE, PINK AND BROWN, hence the name VERSICOLOR, which means "OF VARIOUS COLORS", usually presented From the scalp area, down the neck shoulder, thorax and arms. In some cases itchy or pruritus, but mostly they are asymptomatic.
CONTAGIUM FORMS: DIRECT CONTACT from person to person, POOLS, BEACHES, and elements contaminated with the fungus as clothes and bath towels.
DIAGNOSIS OF PITYRIASIS VERSICOLOR:
====================================
1.) CLINICALLY: the appearance of the lesions.
2.) BESNIER SIGN: Scraping them with the nail causes desquamation.
3.) ADHESIVE TAPE: Consists of placing a transparent "SCOTCH" tape on the lesion, removing it, gluing it to a glass sheet and observing under the light microscope the forms of the fungus described as "spaghetti with meatballs"
4.) KOH: 10% POTASSIUM HYDROXIDE: or PARKER BLUE ink, which is placed on the edges of the tape attached to the sheet, this allows better visualization of the fungus.
5.) WOOD LIGHT: Old method dating back to 1.925 when scientists MARGAROT and DEVEZE discovered that ultraviolet light with a WOOD filter made it possible to see "FLUORESCENCE" in some diseases, in this case: when placing the patient in a dark room and light it with the lamp of Wood: the lesions take a "VIOLET" color. This lamp is commonly used today to check "FALSE TICKETS".
5.) CULTIVATION: in the daily practice is not used, its usefulness is from the scientific point of view for the typification of the fungus and its variants, today more than 13 variants of MALASSEZIA are described, being FURFUR AND GLOBOSA, the most Involved in the human.
THE PITYRIASIS VERSICOLOR CAN BE CONFUSED WITH:
1.) LEPROSY.
2.) PITYIRIASIS ALBA.
3.) VITILIGO.
4.) PIYIRIASIS ROSEA
5.) SEBORRHEIC DERMATITIS.
6.) PROGRESSIVE MACULAR HYPOMELANOSIS.
7.) ERYTHRASMA.
8.) SYPHILIS.
Not all people exposed to the fungus are contaminated, and this happens because the CHEMICAL COMPOSITION OF THE SKIN, IS DIFFERENT FROM PERSON TO PERSON, means that there are people who WILL NEVER SUFFER FROM PITYRIASIS VERSICOLOR, even having relatives and people close with the disease, because it’s LIPHIDIC COMPOSITION, of the skin is not related to the fungus.
THE MOST USED TREATMENTS:
===========================
1.) SODIUM HYPOSULPHITE.
2.) TOLNAFTATE.
3.) KETOCONAZOLE TABLETS AND SHAMPOO.
4.) TERBINAFINE.
5.) FLUCONAZOLE.
6.) ITRACONAZOLE.
7.) SERTACONAZOLE.
8.) SALICYLIC ACID
9.) PHENOL.
10.) BIFONAZOLE
11.) SELENIUM SULFIDE SHAMPOO and others.
12.) CYCLOPIROXOLAMINE.
You must understand that once the treatment is done you should expose your skin to the sun: POOL OR BEACH, paradoxically, BECAUSE if the already healthy skin does not receive sunlight, it will take longer to take its original coloration. The fungus inhibits the normal coloration of the skin producing RESIDUAL HYPOCROMY.
This disease, being totally benign, is often RELAPSE WITH FREQUENCY and this is due to the fact that your skin will always be exposed to the fungus in SWIMMING POOLS, BEACHES, PEOPLE or contaminated CLOTHING, and it why its chemical composition as explain it, in the affected people it has affinity By the fungus.
As maintenance after curing the disease I recommend using a good anti-dandruff shampoo containing an antimycotic like KETOCONAZOLE, BIFONAZOLE or SELENIUM SULFIDE, once or twice weekly. This because of the fact that already explained to you, the fungus has its "HABITAT" on the scalp.
In the references the facts, in the photos different forms OF PITYRIASIS VERSICOLOR.
Greetings to all.
Dr. José Lapenta.
REFERENCIAS BIBLIOGRÁFICAS / BIBLIOGRAPHICAL REFERENCES
=========================================================================
2.) Folliculocentric tinea versicolor.
3.) Comparative efficacy of topical application of tacrolimus and clotrimazole in the treatment of pityriasis versicolor: A single blind, randomised clinical trial.
4.) Comparative clinical trial: fluconazole alone or associated with topical ketoconazole in the treatment of pityriasis versicolor.
6.) Comparison between fluconazole and ketoconazole effectivity in the treatment of pityriasis versicolor.
7.) Treatment of pityriasis versicolor with topical application of essential oil of Cymbopogon citratus (DC) Stapf - therapeutic pilot study.
8.) Atrophying pityriasis versicolor as an idiosyncratic T cell-mediated response to Malassezia: A case series.
9.) Progress in Malassezia Research in Korea.
10.) Malassezia species in healthy skin and in dermatological conditions.
11.) Targeting Malassezia species for novel synthetic and natural antidandruff agents.
12.) Biofilm, adherence, and hydrophobicity as virulence factors in Malassezia furfur.
13.) Antifungal susceptibility testing of Malassezia spp. with an optimized colorimetric broth microdilution method.
14.) Pityriasis versicolor: an update on pharmacological treatment options.
15.) Tinea versicolor and Pityrosporum orbiculare: mycological investigations, experimental infections and epidemiological surveys.
16.) Tinea versicolor and Pityrosporum orbiculare: mycological investigations, experimental infections and epidemiological surveys.
17.) Mycological Considerations in the Topical Treatment of Superficial Fungal Infections.
18.) Single-dose fluconazole versus itraconazole in pityriasis versicolor.
19.) Itraconazole in tinea versicolor: a review.
20.) [Cutaneous Malassezia infections and Malassezia associated dermatoses: An update].
21.) A double-blind comparative study of sodium sulfacetamide lotion 10% versus selenium sulfide lotion 2.5% in the treatment of pityriasis (tinea) versicolor.
22.) Selenium sulfide in tinea versicolor: blood and urine levels.
23.) New Antifungal Agents and New Formulations Against Dermatophytes.
24.) Tavaborole, Efinaconazole, and Luliconazole: Three New Antimycotic Agents for the Treatment of Dermatophytic Fungi.
25.) Therapeutic efficacy and safety of the new antimycotic sertaconazole in the treatment of Pityriasis versicolor.
25.) Whole genome sequencing analysis of the cutaneous pathogenic yeast Malassezia restricta and identification of the major lipase expressed on the scalp of patients with dandruff.
26.) Rook, Arthur et all. Textbook of Dermatology. Blakwell Scientific Publicat. Oxford and Edinburgh 1968.
====================================================================