LIQUEN ESTRIADO Y NEVIL ?
NEVUS EPIDÉRMICO VERRUGOSO INFLAMATORIO LINEAL !!!
LICHEN STRIATUS AND ILVEN?
INFLAMMATORY LINEAR VERRUCOUS EPIDERMAL NEVUS !!!
EDITORIAL ESPAÑOL
==================
Hola amigos de la red DERMAGIC EXPRESS hoy te va hablar de un tema
bastante controversial y discutido hoy día, LA LESIONES
LINEALES en los NIÑOS, específicamente el NEVIL cuyas siglas
significan
NEVUS
EPIDÉRMICO
VERRUGOSO INFLAMATORIO LINEAL, algunos científicos lo denominan
LIQUEN ESTRIADO, otros consideran que son entidades diferentes.
Para aclarar esta controversia vamos a explicar cada una de ellos
por separado, su HISTORIA, ETIOLOGÍA, EVOLUCIÓN, DIAGNÓSTICOS DIFERENCIALES y
TRATAMIENTOS:
1.) LIQUEN ESTRIADO:
A.) HISTORIA:
- Esta patología fue descrita inicialmente en 1898 por dos
dermatólogos franceses:
François Henri Balzer y
Lucien Marie François Mercier
quienes le dieron el nombre de
"trophoneurose lichenoid" en
casos lineales infantiles.
- Posteriormente en 1941, dos dermatólogos estadounidenses:
Francis Eugene Senear, y William Harry Caro, le dieron el nombre definitivo de
"lichen striatus" (liquen estriado),
estandarizándolo clínicamente.
B.) ETIOLOGÍA:
- La causa es desconocida (idiopática), por lo general encuentras
como antecedentes, LESIONES NEVICAS (LUNARES) en los
ascendientes del afectado.
- Como teoría principal de la aparicion del LIQUEN ESTRIADO
se postula un mecanismo denominado
MOSAICISMO GENÉTICO, mas un
DESENCADENAN TE AMBIENTAL, que activa clones de células cutáneas anómalas, en las
líneas de Blaschko, generando una respuesta inflamatoria T-celular, provocando una
migración de queratinocitos hacia esas LINEAS DE BLASCHKO, con la consecuente aparición de las lesiones.
"LAS LINEAS DE BLASCHKO: Son patrones cutáneos invisibles que representan trayectorias de una migración clonal de células epidérmicas que ocurre durante el desarrollo embrionario (semanas 6-8 de gestación). Son lineas imaginarias producto de un MOSAISCIMO GENÉTICO POSTZIGOTICO que siguen la proliferación de DOS poblaciones celulares formando configuraciones dependiendo del area del cuerpo, y no corresponden a dermatomas, ni a vasos linfáticos. Resumiendo es una migración celular embrionaria."
- En S: en el abdomen.
- En V: en la espalda.
- En forma espiral: Tronco.
- Lineales curvas: brazos y piernas.
- Estas Lineas fueron descritas por vez primera en 1901, por el Dermatólogo Alemán Alfred Blaschko en el VII Congreso de la Sociedad Dermatológica Alemana, donde presento 140 casos de lesiones lineales.
LINEAS DE BLASCHKO
En estas lineas IMAGINARIAS, es donde se expresan o manifiestan el LIQUEN ESTRIADO, EL NEVUS VERRUGOSO INFLAMATORIO LINEAL (NEVIL), y otras patologías lineales como LA PSORIASIS LINEAL, el LIQUEN PLANO LINEAL, el VITILIGO SEGMENTARIO, y otras como la INCONTINENCIA PIGMENTARIA.
"EL DERMATOMA: Es un área específica de piel inervada sensorialmente por un solo nervio espinal (raíz dorsal de un segmento medular), ejemplo el CLÁSICO HERPES ZOSTER (NO LINEAL) y el HERPES SIMPLE RECIDIVANTE."
- FACTORES DESENCADENANTES IDENTIFICADOS:
en un 20 a 50% de los casos se han identificado, como
desencadenantes:
a.- Traumatismos menores:
El cual desencadenaría el fenómeno isomorfico de Koebner):
15%.
b.- Infecciones virales (varicela-zóster, EBV, CMV): 30%.
c.- Dermatitis atópica predisponente: 50-61%: historia personal o familiar.
e.- Vacunaciones: aspecto dudoso.
f.- Estrés, medicamentos, y dermatitis por contacto: Raros.
b.- Infecciones virales (varicela-zóster, EBV, CMV): 30%.
c.- Dermatitis atópica predisponente: 50-61%: historia personal o familiar.
e.- Vacunaciones: aspecto dudoso.
f.- Estrés, medicamentos, y dermatitis por contacto: Raros.
C.) CARACTERÍSTICAS CLÍNICAS :
- Se trata de una afección dermatológica rara que se presenta
predominantemente en niños de 4 años en adelante, con promedio entre 1 a
6 años; yo lo he visto aparecer en niños con pocos meses de nacido.
- Se presenta tanto en varones como hembras, con un predominio en
varones 2:1 sobre las niñas.
- Básicamente la lesión está caracterizada por la aparición ESPONTANEA de pápulas planas y placas cuyo color puede variar entre
hipocromía (sin color) o rosadas y en algunos casos hiperpigmentadas,
algunas de aspecto verrugoso, las cuales por cambios de temperatura se
"notan" mas.
- Estas van extendiéndose en forma "LINEAL" siguiendo una metamera de la piel, denominadas LINEAS DE BLASCHKO, hasta formar una especie de "CAMINO" o trayecto lineal, completamente asintomático o levemente
pruriginoso, el cual Puede presentarse en cuello, brazos, piernas, abdomen, área peri-anal, peri-vulvar, y en
algunos casos la cara.
- En algunos casos cuando el LIQUEN ESTRIADO cuando afecta la punta del dedo puede haber afectacion ungueal: onicolisis, onocomadesis, deshilachado. Esto se presenta solo en un 5% de los casos, y por lo general se resuelven las lesiones espontaneamente o con esteroides topicos.
- La mayoría son
totalmente asintomáticas
pero en algunos casos el único síntoma existente es el prurito o comezón
y constituyen un verdadero dolor de cabeza, para los padres de estos
niños que manifiestan esta condición, principalmente por el
aspecto estético.
"Los padres que van a la consulta creen que se trata de una "CULEBRILLA" por el aspecto lineal de la lesión, y una vez explicado el caso, quedan TOTALMENTE SORPRENDIDOS de que este tipo de enfermedad exista en la dermatología"
D.) EVOLUCIÓN:
El LIQUEN ESTRIADO en su evolución, es
AUTO-LIMITADO: El inicio por
lo general es agudo comienza a aparecer en días, con un pico de 1-3
meses, hasta que se detiene la aparición de las lesiones.
- Luego comienza la resolución la cual es
ESPONTANEA, y puede durar entre 6-12 meses (media 9 meses), dejando en
algunos casos
hipopigmentación
residual (28%), o
hiperpigmentación (8%), la cual desaparece en 1-2 años. El tiempo de resolución
disminuye si se aplican tratamientos adecuados.
- La patología raramente recidiva o recae: se dice que un 10% de los
casos; particularmente
NO HE VISTO RECAÍDAS en
estos casos.
E.) TRATAMIENTOS:
Hay varias opciones de tratamiento, que incluyen:
B.- Tacrolimus.
C.- Pimecrolimus.
D.- Calcipotriol.
E. Ttriamcinolona.
F.- Tazaroteno.
G.- Urea.
H. Ácido salicílico.
EL CLÁSICO LIQUEN ESTRIADO: DESAPARECE con un buen tratamiento dermatológico y un buen porcentaje (70%)
espontáneamente.
G.) DIAGNÓSTICOS DIFERENCIALES:
Existe una gran controversia con respecto a estas LESIONES LINEALES en
los niños y adultos y voy a tratar de explicártelo lo mejor posible: Las
mas comunes son:
1.) EL NEVIL O NEVUS EPIDÉRMICO INFLAMATORIO LINEAL:
A.) HISTORIA:
- Esta patología fue descrita
por primera vez en 1894 por el
dermatólogo
Aleman Paul Gerson Unna, uno de
los pioneros de la histopatología cutánea, quien identificó
características psoriasiformes en nevus epidérmicos lineales.
- En 1971, dos dermatologos
estadounidenses: Altman y Mehregan, definieron el término
"NEVUS EPIDÉRMICO VERRUGOSO INFLAMATORIO LINEAL (NEVIL)"
tras serie de 25 casos, diferenciándolo por inflamación intensa, de
otras patologías entre ellas el
LIQUEN ESTRIADO.
B.) ETIOLOGÍA:
NEVUS EPIDÉRMICO VERRUGOSO INFLAMATORIO LINEAL (NEVIL o ILVEN en inglés), tiene etiología similar al LIQUEN ESTRIADO: Se trata de un MOSAICISMO GENETICO, caracterizado por mutaciones postzigóticas en los QUERATINOCITOS durante embriogénesis (semanas 6-8), lo cual provoca una proliferación clonal hamartomatosa, mas una inflamación crónica. El cromosoma alterado es el 15q.
- No es hereditario en el 99% de los casos, considerándose de aparición adquirida esporádica, aunque han sido reportado algunos casos familiares (madre-hija).
- Como factores desencadenantes se han reportado: trauma local y HIV.
C.) CARACTERÍSTICAS CLÍNICAS:
- Es exactamente igual en presentación y características al LIQUEN ESTRIADO, de hecho hay científicos como lo mencione al principio que consideran ambas la misma enfermedad con diferente expresión clínica.
C.) CARACTERÍSTICAS CLÍNICAS:
- Es exactamente igual en presentación y características al LIQUEN ESTRIADO, de hecho hay científicos como lo mencione al principio que consideran ambas la misma enfermedad con diferente expresión clínica.
- Se presenta en niños y adolescentes entre 5 y 15 años y se han descrito casos de presentación en adultos (5%); el 75% de los casos se presenta antes de los 7 años. Un 15% se presenta entre el 1er año de vida y los 5 años.
- Algunos autores consideran la patología de índole congénita.
- Es considerado una variante del NEVUS EPIDÉRMICO, y muy SIMILAR en presentación al LIQUEN ESTRIADO, porque al igual que este, sigue las LINEAS DE BLASCHKO. y los sitios de aparición del mismo son las del Liquen Estriado: cuello, brazos, piernas, abdomen, área peri-anal, peri-vulvar, y en
algunos casos la cara.
D.) EVOLUCIÓN:
La evolución del NEVUS EPIDÉRMICO VERRUGOSO INFLAMATORIO LINEAL (NEVIL) es crónica y progresiva, comenzando en infancia precoz y persistiendo indefinidamente sin remisión espontánea, las lesiones verrugosas persisten y se necesita tratamiento para disminuir su apariencia, o hacerlas desaparecer.
- Hay casos de NEVIL donde las papulas o placas verrugosas NO EXISTEN, pero lo que le da la característica de NEVUS INFLAMATORIO LINEAL es que las lesiones SIN tratamiento NO DESAPARECEN, no hay regresión espontanea. Quedan lesiones hipo-pigmentadas, hiperpigmentadas o acrómicas de por vida.
- Esta es una de las GRANDES diferencias con EL LIQUEN ESTRIADO, el cual si presenta REMISIÓN ESPONTANEA.
E.) TRATAMIENTOS:
A.) Corticosteroides tópicos (40%): poca efectividad.
B.) Calcipotriol: (70%): control parcial.
C.) LASER CO2: (85%): ablación definitiva.
D.) Retinoides sistémicos (60%): poco uso, solo aclara las lesiones.
F.) RESUMEN:
Tanto el LIQUEN ESTRIADO como el NEVUS EPIDÉRMICO VERRUGOSO INFLAMATORIO LINEAL (NEVIL), son muy similares en cuanto a aparición y características clínicas, pero la gran diferencia entre ambas patologías, es lo que ya comentamos. EL LIQUEN ESTRIADO presenta en la gran mayoría de los casos con o sin tratamiento RESOLUCIÓN ESPONTANEA, el NEVUS VERRUGOSO EPIDÉRMICO INFLAMATORIO LINEAL, no presenta REMISIÓN ESPONTANEA, perdura toda la vida, a menos que se haga tratamiento, Y AUN ASÍ, puede que queden marcas residuales.
- Algunos autores consideran ambas patologías una misma ENTIDAD con expresión clínica diferente.
- Algunos científicos han descrito desaparición TOTAL de NEVIL con tratamientos tópicos; este hecho plantea la gran interrogante ? eran LIQUEN ESTRIADOS ??
- Aquí te voy a decir algo basado en MI EXPERIENCIA: EL LIQUEN ESTRIADO, considerado por algunos científicos como una variante del NEVIL (NEVUS VERRUGOSO INFLAMATORIO LINEAL), cuando desaparece con un buen tratamiento
dermatológico, o espontáneamente, NO VUELVE A APARECER. JAMAS he visto un LIQUEN ESTRIADO que se curó, volver a presentarse, o recidivar.
NEVUS EPIDÉRMICO VERRUGOSO INFLAMATORIO LINEAL
EL NEVUS EPIDÉRMICO VERRUGOSO LINEAL: (NO INFLAMATORIO):
Se trata del CLÁSICO NEVIL: una lesión NEVICA, tipo lunar en LINEA de aspecto VERRUGOSO, (ver foto), su coloración es pardo
claro o hiperpigmentada. Como dijimos previamente, la gran diferencia es que
este NO DESAPARECE con tratamiento tópico convencional y persiste hasta edad
adulta. Hay que recurrir a otros métodos como la extirpación
quirúrgica o laser, la mayoría de ellos son congénitos o aparecen
en la infancia.
NEVUS EPIDÉRMICO VERRUGOSO LINEAL DORSO DE MANO
DIAGNOSTICO DIFERENCIAL
CON OTRAS PATOLOGÍAS CUTÁNEAS LINEALES:
1.) PSORIASIS SEGMENTADA O LINEAL:
Hay muchos científicos que consideran al NEVIL como una variante
de la PSORIASIS, denominándola PSORIASIS LINEAL,
basados fundamentalmente en los hallazgos HISTOPATOLÓGICO (BIOPSIA) de las lesiones.
Por otra parte la PSORIASIS como todos ustedes saben es una
enfermedad multi factorial, que da signos y síntomas en otras aéreas
del cuerpo, y que tiende a recaer a menudo, relacionada con
VITILIGO y DIABETES. La mayoría de los niños con NEVIL o LIQUEN ESTRIADO que he
tratado NUNCA presentaron SIGNOS O SÍNTOMAS DE PSORIASIS, ni
recayeron, de modo que:
EL NEVIL Y EL LIQUEN ESTRIADO son totalmente diferentes a LA PSORIASIS LINEAL O SEGMENTADA, SON ENTIDADES DIFERENTES: La psoriasis lineal también sigue a las LINEAS DE BLASHCKO, pero clínicamente son las clásicas placas eritemato-escamosas TÍPICAS de la PSORIASIS; esta patología responde a tratamientos tópicos y sistémicos.
- Por cierto, NUNCA HE VISTO UN CASO DE "PSORIASIS LINEAL", en
niños ni adultos, existe como entidad, variante de la
PSORIASIS, pero no tiene nada que ver con el clásico NEVIL y LIQUEN ESTRIADO, que se
presenta en niño, y ocasionalmente en adultos.
2.) LIQUEN PLANO LINEAL:
LIQUEN PLANO LINEAL PIGMENTADO DEL CUELLO
3.) NEVUS SEBACEO LINEAL:
Esta lesión se presenta fundamentalmente en el cuero cabelludo, pero
también se puede presentar ocasionalmente en otras aéreas de la cara
descrito por primera vez por Josef Jadassohn, en 1895, medico Suizo alemán en conocido también como NEVUS SEBACEO DE JADASSOHN, es de aspecto verrugoso color pardo o anaranjado, congénito (está presente al
nacer), y su tratamiento es principalmente quirúrgico. (Ver foto)
NEVUS SEBACEO LINEAL (DE JADASSONHN)
4.) VITILIGO SEGMENTADO:
Es una variante del clásico vitiligo caracterizado por
maculas ACROMICAS (SIN COLOR) que aparecen en una metamera del cuerpo siguiendo las LINEAS DE BLASCHKO, son de mayor tamaño que las lesiones del
clásico LIQUEN ESTRIADO y NEVIL; puede presentarse en niños como adultos y el
tratamiento en estos casos es mas difícil pues la piel perdió la
coloración a nivel de los MELANOCITOS encargados de producir el pigmento o color de la piel.
5.) HERPES ZOSTER: (Virus de la Varicela-Zóster/VVZ)
EL HERPES ZOSTER, se presenta como un TRAYECTO LINEAL, rampante ("culebrilla"), el cual es adquirido, y producido por el virus de la VARICELA ZOSTER (VVZ), y la característica de las lesiones son ampollas y vesículas, acompañadas de prurito y dolor. Puede presentarse en cualquier area del cuerpo, incluyendo cuero cabelludo y mucosas (ojos), genitales.
- De modo que es totalmente diferente del LIQUEN ESTRIADO y NEVIL; la lesion desaparece con tratamiento medico, dejando en muchos casos un gran dolor, denominado NEURALGIA POST HERPETICA.(ACTUALIZACIÓN).
G.) CONCLUSIONES: si tu niño comienza a presentar lesiones dermatológicas en
forma de "LINEA" llévalo a la consulta dermatológica para establecer el
diagnostico adecuado y realizar un buen tratamiento.
Si eres adulto igualmente debes consultar a tu dermatólogo
- Repetimos: el LIQUEN ESTRIADO y EL NEVUS EPIDÉRMICO LINEAL (NEVIL), son muy parecidos.
Espero que esta revisión bibliográfica y fotos te orienten al
respecto.
Saludos a todos.
Dr. José M. Lapenta
EDITORIAL ENGLISH
===================
Hello friends of the DERMAGIC EXPRESS network today you I´m going
to talk about a very controversial topic and discussed today, THE LINEAR SKIN LESIONS in CHILDREN, specifically the
ILVEN whose initials stand for INFLAMMATORY LINEAR VERRUCOUS EPIDERMIC NEVUS, some scientists call it LICHEN STRIATUS, and others consider that
they are Different entities.
To clarify this controversy we are going to explain each one of them separately, their HISTORY, ETIOLOGY, EVOLUTION, DIFFERENTIAL DIAGNOSES and TREATMENTS:
1.) STRIATUS LICHEN:
A.) HISTORY:
This pathology was initially described in 1898 by two French dermatologists: François Henri Balzer and Lucien Marie François Mercier who gave it the name "trophoneurose lichenoid" in linear childhood cases.
- Subsequently in 1941, two American dermatologists: Francis Eugene Senear, and William Harry Caro, gave it the definitive name of "lichen striatus" (striated lichen), standardizing it clinically.
B.) ETIOLOGY:
1.) STRIATUS LICHEN:
A.) HISTORY:
This pathology was initially described in 1898 by two French dermatologists: François Henri Balzer and Lucien Marie François Mercier who gave it the name "trophoneurose lichenoid" in linear childhood cases.
- Subsequently in 1941, two American dermatologists: Francis Eugene Senear, and William Harry Caro, gave it the definitive name of "lichen striatus" (striated lichen), standardizing it clinically.
B.) ETIOLOGY:
- The cause is unknown (idiopathic), you usually find as background, NEVIC LESIONS (MOLES) in the ancestors of the affected.
- As the main theory of the appearance of STRIATUS LICHEN, a mechanism called GENETIC MOSAICISM is postulated, plus an ENVIRONMENTAL TRIGGER, that activates clones of anomalous skin cells, in the lines of Blaschko, generating a T-cell inflammatory response, causing a migration of keratinocytes towards those LINES OF BLASCHKO, with the consequent appearance of the lesions.
In S: in the abdomen.
In V: in the back.
In spiral form: Trunk.
Curved linears: arms and legs.
- These Lines were described for the first time in 1901, by the German Dermatologist Alfred Blaschko in the VII Congress of the German Dermatological Society, where he presented 140 cases of linear lesions.
"THE LINES OF BLASCHKO: They are invisible cutaneous patterns that represent trajectories of a clonal migration of epidermal cells that occurs during embryonic development (weeks 6-8 of gestation). They are imaginary lines product of a POSTZYGOTIC GENETIC MOSAICISM that follow the proliferation of TWO cell populations forming configurations depending on the body area, and do not correspond to dermatomes, nor to lymphatic vessels. Summarizing it is an embryonic cell migration."
In S: in the abdomen.
In V: in the back.
In spiral form: Trunk.
Curved linears: arms and legs.
- These Lines were described for the first time in 1901, by the German Dermatologist Alfred Blaschko in the VII Congress of the German Dermatological Society, where he presented 140 cases of linear lesions.
BLASCHKO'S LINES
In these IMAGINARY LINES, is where LICHEN STRIATUS, THE LINEAR INFLAMMATORY VERRUCOUS NEVUS (ILVEN), and other linear pathologies such as LINEAR PSORIASIS, LINEAR LICHEN PLANUS, SEGMENTAL VITILIGO, and others like INCONTINENCE PIGMENTARY are expressed or manifested.
"THE DERMATOME: It is a specific area of skin sensorially innervated by a single spinal nerve (dorsal root of a medullary segment), example the CLASSIC HERPES ZOSTER (NOT LINEAR) and RECURRENT SIMPLE HERPES."
IDENTIFIED TRIGGERING FACTORS: in 20 to 50% of cases have been identified, as triggers:
a.- Minor traumas: Which would trigger the isomorphic phenomenon of Koebner): 15%.
b.- Viral infections: (varicella-zoster, EBV, CMV): 30%.
c.- Predisposing atopic dermatitis:50-61%: personal or family history.
e.- Vaccinations: doubtful aspect.
f.- Stress, medications, and contact dermatitis: Rare.
C.) CLINICAL CHARACTERISTICS:
- It is a rare dermatological condition that predominantly presents in children from 4 years onwards, with an average between 1 to 6 years; I have seen it appear in children a few months old.
- It presents in both males and females, with a predominance in males 2:1 over girls.
a.- Minor traumas: Which would trigger the isomorphic phenomenon of Koebner): 15%.
b.- Viral infections: (varicella-zoster, EBV, CMV): 30%.
c.- Predisposing atopic dermatitis:50-61%: personal or family history.
e.- Vaccinations: doubtful aspect.
f.- Stress, medications, and contact dermatitis: Rare.
C.) CLINICAL CHARACTERISTICS:
- It is a rare dermatological condition that predominantly presents in children from 4 years onwards, with an average between 1 to 6 years; I have seen it appear in children a few months old.
- It presents in both males and females, with a predominance in males 2:1 over girls.
- Basically the lesion is characterized by the SPONTANEOUS appearance of flat papules and plaques whose color can vary between hypochromia (no color) or pink and in some cases hyperpigmented, some with a verrucous appearance, which by temperature changes are "noticed" more.
- These spread in a "LINEAR" way following a metamer of the skin, called LINES OF BLASCHKO, until forming a kind of "PATH" or linear trajectory, completely asymptomatic or mildly pruritic, which Can present in neck, arms, legs, abdomen, peri-anal area, peri-vulvar, and in some cases the face.
- In some cases when LICHEN STRIATUS affects the tip of the finger there may be nail involvement: onycholysis, onychomadesis, sliptting. This occurs only in 5% of cases, and the lesions generally resolve spontaneously or with topical steroids.
- Most are totally asymptomatic but in some cases the only existing symptom is pruritus or itching and constitute a real headache, for the parents of these children who manifest this condition, mainly due to the aesthetic appearance.
"Parents who go to the consultation believe it is a "SHINGLES" due to the linear appearance of the lesion, and once the case is explained, they are TOTALLY SURPRISED that this type of disease exists in dermatology"
"Parents who go to the consultation believe it is a "SHINGLES" due to the linear appearance of the lesion, and once the case is explained, they are TOTALLY SURPRISED that this type of disease exists in dermatology"
D.) EVOLUTION:
LICHEN STRIATUS in its evolution, is SELF-LIMITED: The onset is generally acute begins to appear in days, with a peak of 1-3 months, until the appearance of the lesions stops.
- Then the resolution begins which is SPONTANEOUS, and can last between 6-12 months (average 9 months), leaving in some cases residual hypopigmentation (28%), or hyperpigmentation (8%), which disappears in 1-2 years. The resolution time decreases if appropriate treatments are applied.
- The pathology rarely relapses or recurs: it is said that 10% of cases; particularly I HAVE NOT SEEN RELAPSES in these cases.
E.) TREATMENTS:
There are several treatment options, which include:
A.- Topical corticosteroids.
B.- Tacrolimus.
C.- Pimecrolimus.
D.- Calcipotriol.
E. Triamcinolone.
F.- Tazarotene.
G.- Urea.
H. Salicylic acid.
THE CLASSIC LICHEN STRIATUS: DISAPPEARS with good dermatological treatment and a good percentage (70%) spontaneously.
G.) DIFFERENTIAL DIAGNOSES:
There is a great controversy regarding these LINEAR LESIONS in children and adults and I am going to try to explain it to you as best as possible: The most common are:
1.) THE ILVEN OR LINEAR INFLAMMATORY EPIDERMAL NEVUS:
A.) HISTORY:
- This pathology was described for the first time in 1894 by the German dermatologist Paul Gerson Unna, one of the pioneers of cutaneous histopathology, who identified psoriasiform characteristics in linear epidermal nevi.
- In 1971, two American dermatologists: Altman and Mehregan, defined the term "LINEAR INFLAMMATORY VERRUCOUS EPIDERMAL NEVUS (ILVEN)" after a series of 25 cases, differentiating it by intense inflammation, from other pathologies including STRIATUS LICHEN.
B.) ETIOLOGY:
LINEAR INFLAMMATORY VERRUCOUS EPIDERMAL NEVUS (or ILVEN in English), has etiology similar to LICHEN STRIATUS: It is a GENETIC MOSAICISM, characterized by postzygotic mutations in KERATINOCYTES during embryogenesis (weeks 6-8), which causes a clonal hamartomatous proliferation, plus chronic inflammation. The altered chromosome is 15q.
- It is not hereditary in 99% of cases, considered sporadic acquired appearance, although some familial cases (mother-daughter) have been reported.
- As triggering factors have been reported: local trauma and HIV.
C.) CLINICAL CHARACTERISTICS:
- It is exactly the same in presentation and characteristics as LICHEN STRIATUS, in fact there are scientists as I mentioned at the beginning who consider both the same disease with different clinical expression.
- It presents in children and adolescents between 5 and 15 years and cases of presentation in adults have been described (5%); 75% of cases present before 7 years. 15% present between the 1st year of life and 5 years.
- Some authors consider the pathology of congenital nature.
- It is considered a variant of the EPIDERMAL NEVUS, and very SIMILAR in presentation to LICHEN STRIATUS, because like this one, it follows the LINES OF BLASCHKO. and the sites of appearance are the same as Lichen striatus: neck, arms, legs, abdomen, peri-anal area, peri-vulvar, and in some cases the face.
D.) EVOLUTION:
- The evolution of LINEAR INFLAMMATORY VERRUCOUS EPIDERMAL NEVUS (ILVEN) is chronic and progressive, beginning in early childhood and persisting indefinitely without spontaneous remission, the verrucous lesions persist and treatment is needed to reduce their appearance, or make them disappear.
- There are cases of ILVEN where the papules or verrucous plaques DO NOT EXIST, but what gives it the characteristic of LINEAR EPIDERMAL INFLAMMATORY NEVUS is that the lesions WITHOUT treatment DO NOT DISAPPEAR, there is no spontaneous regression. Hypo-pigmented, hyperpigmented or achromic lesions remain for life.
B.) ETIOLOGY:
LINEAR INFLAMMATORY VERRUCOUS EPIDERMAL NEVUS (or ILVEN in English), has etiology similar to LICHEN STRIATUS: It is a GENETIC MOSAICISM, characterized by postzygotic mutations in KERATINOCYTES during embryogenesis (weeks 6-8), which causes a clonal hamartomatous proliferation, plus chronic inflammation. The altered chromosome is 15q.
- It is not hereditary in 99% of cases, considered sporadic acquired appearance, although some familial cases (mother-daughter) have been reported.
- As triggering factors have been reported: local trauma and HIV.
C.) CLINICAL CHARACTERISTICS:
- It is exactly the same in presentation and characteristics as LICHEN STRIATUS, in fact there are scientists as I mentioned at the beginning who consider both the same disease with different clinical expression.
- It presents in children and adolescents between 5 and 15 years and cases of presentation in adults have been described (5%); 75% of cases present before 7 years. 15% present between the 1st year of life and 5 years.
- Some authors consider the pathology of congenital nature.
- It is considered a variant of the EPIDERMAL NEVUS, and very SIMILAR in presentation to LICHEN STRIATUS, because like this one, it follows the LINES OF BLASCHKO. and the sites of appearance are the same as Lichen striatus: neck, arms, legs, abdomen, peri-anal area, peri-vulvar, and in some cases the face.
D.) EVOLUTION:
- The evolution of LINEAR INFLAMMATORY VERRUCOUS EPIDERMAL NEVUS (ILVEN) is chronic and progressive, beginning in early childhood and persisting indefinitely without spontaneous remission, the verrucous lesions persist and treatment is needed to reduce their appearance, or make them disappear.
- There are cases of ILVEN where the papules or verrucous plaques DO NOT EXIST, but what gives it the characteristic of LINEAR EPIDERMAL INFLAMMATORY NEVUS is that the lesions WITHOUT treatment DO NOT DISAPPEAR, there is no spontaneous regression. Hypo-pigmented, hyperpigmented or achromic lesions remain for life.
- This is one of the GREAT differences with LICHEN STRIATUS, which does present SPONTANEOUS REMISSION.
E.) TREATMENTS:
A.) Topical corticosteroids (40%): little effectiveness.
B.) Calcipotriol: (70%): partial control.
C.) CO2 LASER: (85%): definitive ablation.
D.) Systemic retinoids (60%): little use, only lightens the lesions.
F.) SUMMARY:
Both LICHEN STRIATUS and LINEAR INFLAMMATORY VERRUCOUS EPIDERMAL NEVUS (ILVEN), are very similar in terms of appearance and clinical characteristics, but the great difference between both pathologies, is what we already discussed. LICHEN STRIATUS presents in the vast majority of cases with or without treatment SPONTANEOUS RESOLUTION, the LINEAR INFLAMMATORY VERRUCOUS EPIDERMAL NEVUS, does not present SPONTANEOUS REMISSION, it lasts a lifetime, unless treatment is done, AND EVEN SO, residual marks may remain.
E.) TREATMENTS:
A.) Topical corticosteroids (40%): little effectiveness.
B.) Calcipotriol: (70%): partial control.
C.) CO2 LASER: (85%): definitive ablation.
D.) Systemic retinoids (60%): little use, only lightens the lesions.
F.) SUMMARY:
Both LICHEN STRIATUS and LINEAR INFLAMMATORY VERRUCOUS EPIDERMAL NEVUS (ILVEN), are very similar in terms of appearance and clinical characteristics, but the great difference between both pathologies, is what we already discussed. LICHEN STRIATUS presents in the vast majority of cases with or without treatment SPONTANEOUS RESOLUTION, the LINEAR INFLAMMATORY VERRUCOUS EPIDERMAL NEVUS, does not present SPONTANEOUS REMISSION, it lasts a lifetime, unless treatment is done, AND EVEN SO, residual marks may remain.
- Some authors consider both pathologies the same ENTITY with different clinical expression.
- Some scientists have described TOTAL disappearance of NEVIL with topical treatments; this fact raises the great question ? were they LICHEN STRIATUS ??
- Some scientists have described TOTAL disappearance of NEVIL with topical treatments; this fact raises the great question ? were they LICHEN STRIATUS ??
- Here I am going to tell you something based on MY EXPERIENCE: LICHEN STRIATUS , considered by some scientists as a variant of ILVEN (LINEAR INFLAMMATORY VERRUCOUS NEVUS), when it disappears with good dermatological treatment, or spontaneously, DOES NOT REAPPEAR. I HAVE NEVER seen a LICHEN STRIATUS that was cured, reappear, or relapse.
LINEAR INFLAMMATORY VERRUCOUS EPIDERMAL NEVUS
THE LINEAR VERRUCOUS EPIDERMAL NEVUS: (NON INFLAMMATORY):
It is the CLASSIC ILVEN: a NEVIC lesion, mole type in LINE with VERRUCOUS appearance, (see photo), its coloration is light brown or hyperpigmented. As we said previously, the great difference is that this DOES NOT DISAPPEAR with conventional topical treatment and persists into adulthood. Other methods such as surgical excision or laser must be resorted to, most of them are congenital or appear in childhood.
LINEAR VERRUCOUS EPIDERMAL NEVUS BACK OF HAND
DIFFERENTIAL DIAGNOSIS WITH OTHER LINEAR CUTANEOUS PATHOLOGIES:
1.) THE SEGMENTAL PSORIASIS (LINEAR):
There are many scientists who consider ILVEN as a variant of
PSORIASIS, denominating it LINEAR PSORIASIS, based
fundamentally on the HISTOPATHOLOGICAL (BIOPSY) findings of the
lesions.
Here I am going to tell you something based on
MY EXPERIENCE:
ILVEN (INFLAMMATORY LINEAR VERRUCOUS EPIDERMAL NEVUS) it disappears with a good dermatological treatment and
DOES NOT REPEAT or REPLAPSE,
I have NEVER seen an
ILVEN that was cured to relapse
itself.
On the other hand the PSORIASIS as you all know is a multifactorial
disease that gives signs and symptoms in other areas of the body,
and that tends to relapse often, related to VITILIGO AND
DIABETES.
The majority of the children with ILVEN that I have tried NEVER presented SIGNS OR SYMPTOMS OF PSORIASIS, nor they relapse, so that...
The majority of the children with ILVEN that I have tried NEVER presented SIGNS OR SYMPTOMS OF PSORIASIS, nor they relapse, so that...
With great respect to scientists I say that
ILVEN AND
LINEAR OR SEGMENTAL PSORIASIS ARE DIFFERENT ENTITIES,
by the way, I have NEVER SEEN A CASE OF "LINEAR PSORIASIS", in
children or adults, it may exist as an entity, variant of PSORIASIS,
But it has nothing to do with the classic ILVEN that occurs in
children.
LINEAR SEGMENTAL PSORIASIS OF THE NECK
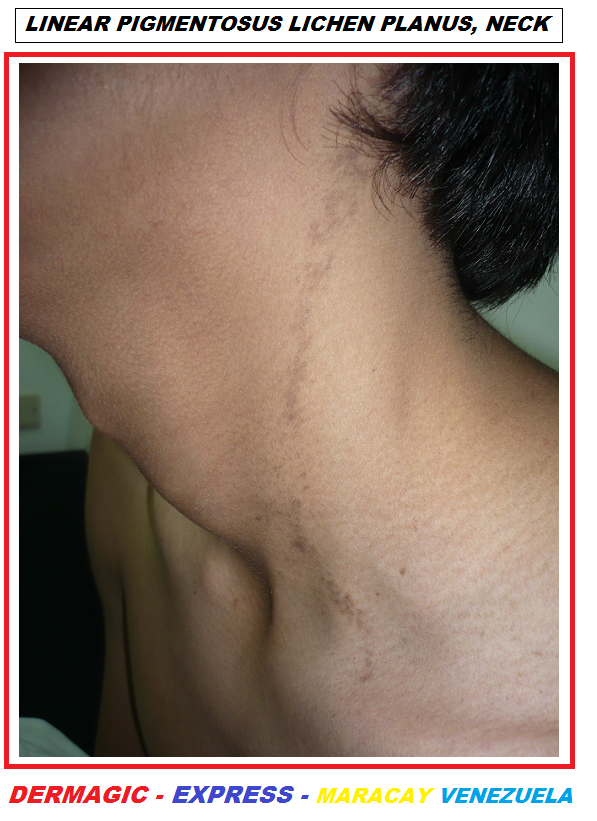
2.) LINEAR LICHEN PLANUS:
The LINEAR pigmentosus lichen planus is another entity that could be
confused with an ILVEN, but this usually occurs in adults and the
color of the lesion is usually a VIOLET or
BROWN
color,
LICHEN PLANUS UPADE 2025 which is highly PRURITIC, may appear on the face, highly related to
everyday stress. But just as ILVEN also disappears with a good
dermatological treatment. (View photo)
3.) SEBACEOUS LINEAR NEVUS:
===========================
This lesion occurs primarily in the scalp, but may also occur
occasionally in other areas of the face first described by Josef Jadassohn, a Swiss-German physician, in 1895, also known as SEBACEOUS NEVUS OF JADASSOHN, is
congenitally
brown or orange-colored (it is present at birth), and its treatment is primarily surgical.
(View photo)
4.) SEGMENTAL VITILIGO:
=======================
It is a variant of the classic vitiligo characterized by
ACROMIC macules (without COLOR) that appear in the body
following the lines of Blaschko, are of greater size than the
classic ILVEN lesions, can present in children as adults and the
treatment in these cases Is more difficult because the skin lost the
coloration at the level of the MELANOCYTES
responsible for producing the pigment or color of the skin.
5.) HERPES ZOSTER: (Varicella-Zoster Virus/VZV)
THE HERPES ZOSTER, presents as a LINEAR TRAJECTORY, rampaging ("shingles"), which is acquired, and produced by the VARICELLA ZOSTER virus (VZV), and the characteristic of the lesions are blisters and vesicles, accompanied by pruritus and pain. It can present in any area of the body, including scalp and mucous membranes (eyes), genitals.
THE HERPES ZOSTER, presents as a LINEAR TRAJECTORY, rampaging ("shingles"), which is acquired, and produced by the VARICELLA ZOSTER virus (VZV), and the characteristic of the lesions are blisters and vesicles, accompanied by pruritus and pain. It can present in any area of the body, including scalp and mucous membranes (eyes), genitals.
- So it is totally different from LICHEN STRIATUS and ILVEN; the lesion disappears with medical treatment, leaving in many cases great pain, called POST-HERPETIC NEURALGIA.(UPDATE).
CONCLUSION: if your
child begins to present dermatological lesions in the form of
"LINE" take it to the
dermatological consultation to establish the proper diagnosis and to
perform a good dermatological treatment. If you are an adult you
should also consult your dermatologist.
- We repeat: LICHEN STRIATUS and THE INFLAMMATORY LINEAR VERRUGOUS EPIDERMAL NEVUS (ILVEN), are very similar.
I hope this bibliographic review and photos guide you !
Greetings to all.
Dr. José Lapenta.
Dr. José M. Lapenta.
Dr. José M. Lapenta.
=======================================================================
REFERENCIAS BIBLIOGRÁFICAS/ BIBLIOGRAPHICAL REFERENCES
=======================================================================
A.- Nail Lichen Striatus and Its Differential Diagnoses (2024).
B.- Lichen striatus: a review (2025).
REFERENCIAS BIBLIOGRÁFICAS/ BIBLIOGRAPHICAL REFERENCES
=======================================================================
A.- Nail Lichen Striatus and Its Differential Diagnoses (2024).
B.- Lichen striatus: a review (2025).
=======================================================================
1.) ILVEN - COMPLETE REMISSION AFTER ADMINISTRATION OF TOPICAL
CORTICOSTEROID (CASE REVIEW).
2.) Successful treatment of inflammatory linear verrucous epidermal nevus with tacrolimus and fluocinonide.
3.) Genital/Perigenital Inflammatory Linear Verrucous Epidermal Nevus: A Case Series.
4.) Does inflammatory linear verrucous epidermal nevus represent a segmental type 1/type 2 mosaic of psoriasis?
5.) Immunohistochemical differentiation between inflammatory linear verrucous epidermal nevus (ILVEN) and psoriasis.
6.) Inflammatory linear verrucous epidermal nevus syndrome with its polymorphic presentation - A rare case report.
7.) Vulval and perianal inflammatory linear verrucous epidermal naevus.
8.) Adult onset of inflammatory linear verrucous epidermal nevus.
9.) Inflammatory linear verrucous epidermal nevus (ILVEN).
10.) Histopathologic varieties of epidermal nevus. A study of 160 cases.
11.) Inflammatory linear verrucose epidermal nevus.
12.) Inflammatory linear verrucous epidermal naevus (ILVEN) versus linear psoriasis. A clinical, histological and immunohistochemical study.
13.) Inflammatory linear verrucous epidermal nevus: why a combined laser therapy.14.) Inflammatory linear verrucous epidermal nevus and arthritis: a new association.
15.) [Successful therapy of an ILVEN in a 7-year-old girl with calcipotriol].
16.) Dithranol in the treatment of inflammatory linear verrucous epidermal nevus.
17.) Naevoid Psoriasis and ILVEN: Same Coin, Two Faces?
18.) Carbon dioxide laser treatment of epidermal nevi: response and long-term follow-up.
19.) A case of linear lichen planus pigmentosus.
20.) Lichen planus pigmentosus presenting in zosteriform pattern.
21.) Differential Diagnosis of Linear Eruptions in Children.
22.) Two cases of lichen striatus with prolonged active phase.
23.) Effective topical combination therapy for treatment of lichen striatus in children: a case series and review.
24.) [Lichen striatus with nail abnormality is a self-limiting condition].
================================================================
2.) Successful treatment of inflammatory linear verrucous epidermal nevus with tacrolimus and fluocinonide.
3.) Genital/Perigenital Inflammatory Linear Verrucous Epidermal Nevus: A Case Series.
4.) Does inflammatory linear verrucous epidermal nevus represent a segmental type 1/type 2 mosaic of psoriasis?
5.) Immunohistochemical differentiation between inflammatory linear verrucous epidermal nevus (ILVEN) and psoriasis.
6.) Inflammatory linear verrucous epidermal nevus syndrome with its polymorphic presentation - A rare case report.
7.) Vulval and perianal inflammatory linear verrucous epidermal naevus.
8.) Adult onset of inflammatory linear verrucous epidermal nevus.
9.) Inflammatory linear verrucous epidermal nevus (ILVEN).
10.) Histopathologic varieties of epidermal nevus. A study of 160 cases.
11.) Inflammatory linear verrucose epidermal nevus.
12.) Inflammatory linear verrucous epidermal naevus (ILVEN) versus linear psoriasis. A clinical, histological and immunohistochemical study.
13.) Inflammatory linear verrucous epidermal nevus: why a combined laser therapy.14.) Inflammatory linear verrucous epidermal nevus and arthritis: a new association.
15.) [Successful therapy of an ILVEN in a 7-year-old girl with calcipotriol].
16.) Dithranol in the treatment of inflammatory linear verrucous epidermal nevus.
17.) Naevoid Psoriasis and ILVEN: Same Coin, Two Faces?
18.) Carbon dioxide laser treatment of epidermal nevi: response and long-term follow-up.
19.) A case of linear lichen planus pigmentosus.
20.) Lichen planus pigmentosus presenting in zosteriform pattern.
21.) Differential Diagnosis of Linear Eruptions in Children.
22.) Two cases of lichen striatus with prolonged active phase.
23.) Effective topical combination therapy for treatment of lichen striatus in children: a case series and review.
24.) [Lichen striatus with nail abnormality is a self-limiting condition].
================================================================